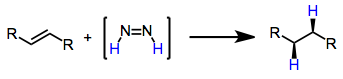|
Reductions With Diimide
Reductions with diimide are a chemical reactions that convert unsaturated organic compounds to reduced alkane products. In the process, diimide () is oxidized to dinitrogen. Introduction In 1929, the conversion of oleic acid to stearic acid in the presence of hydrazine was observed. The short-lived intermediate diimide was not implicated in this reductive process until the 1960s. Since that time, several methods of generating transient amounts of diimide have been developed. In the presence of unpolarized alkenes, alkynes or allenes, diimide is converted into dinitrogen with reduction (net addition of dihydrogen) of the unsaturated functionality. Diimide formation is the rate-limiting step of the process, and a concerted mechanism involving ''cis''-diimide has been proposed. This reduction represents a metal-free alternative to catalytic hydrogenation reductions, and does not lead to the cleavage of sensitive O–O and N–O bonds. Mechanism and stereochemistry Prevailing mechanis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula . The alkanes range in complexity from the simplest case of methane (), where ''n'' = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like hexacontane () or 4-methyl-5-(1-methylethyl) octane, an isomer of dodecane (). The International Union of Pure and Applied Chemistry (IUPAC) defines alkanes as "acyclic branched or unbranched hydrocarbons having the general formula , and therefore consisting entirely of hydrogen atoms and saturated carbon atoms". However, some sources use the term to denote ''any'' saturated hydrocarbon, including those that are either monocyclic (i.e. the cycloalkanes) or polycycl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diimide
Diimide, also called diazene or diimine, is a compound having the formula HN=NH. It exists as two geometric isomers, ''E'' (''trans'') and ''Z'' (''cis''). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an example of an organic diazene. Synthesis A traditional route to diimide involves oxidation of hydrazine with hydrogen peroxide or air. : Alternatively the hydrolysis of diethyl azodicarboxylate or azodicarbonamide affords diimide: : Nowadays, diimide is generated by thermal decomposition of 2,4,6‐triisopropylbenzenesulfonylhydrazide. Because of its instability, diimide is generated and used ''in-situ''. A mixture of both the ''cis'' (''Z-'') and ''trans'' (''E-'') isomers is produced. Both isomers are unstable, and they undergo a slow interconversion. The ''trans'' isomer is more stable, but the ''cis'' isomer is the one that reacts with unsaturated substrates, therefore the equilibrium between them shifts towards the ''cis'' isomer d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oleic Acid
Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish due to the presence of impurities. In chemical terms, oleic acid is classified as a monounsaturated omega-9 fatty acid, abbreviated with a lipid number of 18:1 ''cis''-9, and a main product of Δ9-desaturase. It has the formula . The name derives from the Latin word '' oleum'', which means oil. It is the most common fatty acid in nature. The salts and esters of oleic acid are called oleates. It is a common component of oils, and thus occurs in many types of food, as well as in soap. Occurrence Fatty acids (or their salts) often do not occur as such in biological systems. Instead fatty acids such as oleic acid occur as their esters, commonly triglycerides, which are the greasy materials in many natural oils. Oleic acid is the most common monounsaturated fatty acid in nature. It is found in fats (trigl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stearic Acid
Stearic acid ( , ) is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a soft waxy solid with the formula . The triglyceride derived from three molecules of stearic acid is called stearin. Stearic acid is a prevalent fatty-acid in nature, found in many animal and vegetable fats, but is usually higher in animal fat than vegetable fat. It has a melting point of °C and a pKa of 4.50. Its name comes from the Greek word στέαρ "''stéar''", which means tallow. The salts and esters of stearic acid are called stearates. As its ester, stearic acid is one of the most common saturated fatty acids found in nature and in the food supply, following palmitic acid.Gunstone, F. D., John L. Harwood, and Albert J. Dijkstra "The Lipid Handbook with Cd-Rom. 3rd ed. Boca Raton: CRC Press, 2007. , Dietary sources of stearic acid include meat, poultry, fish, eggs, dairy products, and foods prepared with fats; beef tallow, lard, butterfat, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing Polymeric foam, polymer foams, but applications also include its uses as a precursor (chemistry), precursor to pharmaceuticals and agrochemicals, as well as a long-term storable propellant for in-outer space, space spacecraft propulsion. Additionally, hydrazine is used in various rocket propellant, rocket fuels and to prepare the gas precursors used in airbags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. , approximately 120,000 tons of hydrazine hydrate (corresponding to a 64% solution of hydrazine in water by weight) we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Azodicarboxylate
Potassium azodicarboxylate is a chemical compound with the formula C2K2N2O4. This chemical is used as a precursor to diimide. It can be synthesized by the reaction of potassium hydroxide with azodicarbonamide Azodicarbonamide, ADCA, ACA, ADA, or azo(''bis'')formamide, is a chemical compound with the molecular formula . It is a yellow to orange-red, odorless, crystalline powder. It is sometimes called "the yoga mat chemical" because of widespread use i ... and it reacts with carboxylic acids to form diimide. References {{reflist Potassium compounds Azo compounds Reagents for organic chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |