|
Propalene
Propalene or bicyclo .1.0uta-1,3-diene is a polycyclic hydrocarbon composed of two fused cyclopropene rings. Computational studies indicate that the molecule is planar, with the carbon framework forming a parallelogram that has distinctly alternating short and long carbon–carbon bonds. See also *Butadiene *Cyclobutadiene * Bicyclobutane * Butalene *Pentalene *Heptalene Heptalene is a polycyclic hydrocarbon with chemical formula , composed of two fused cycloheptatriene rings. It is an unstable, non-planar compound which is non-aromatic. The dianion, however, satisfies Hückel's rule In organic chemistry, Hü ... * Octalene References Polycyclic nonaromatic hydrocarbons Bicyclic compounds Cyclopropenes {{Hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butalene
Butalene is a polycyclic hydrocarbon composed of two fused cyclobutadiene rings. A reported possible synthesis of it involves an elimination reaction from a Dewar benzene derivative. The structure itself can be envisioned as benzene with an internal bridge, and calculations indicate it is somewhat less stable than the open 1,4-didehydrobenzene biradical, the valence isomer in which that bridged bond is broken. Structure and bonding Ab initio calculations indicate butalene has a planar geometry and, in keeping with a planar structure with 6 π-electron configuration, is aromatic. Thus, the most significant π bonding interactions involve conjugation around the periphery of the whole six-atom structure, similar to benzene, rather than cross-ring resonance along the bridging bond. Significant resonance around one or the other four-membered ring alone would be a less-stable antiaromatic form, as is seen in cyclobutadiene itself. See also *Propalene *Pentalene *Heptalene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
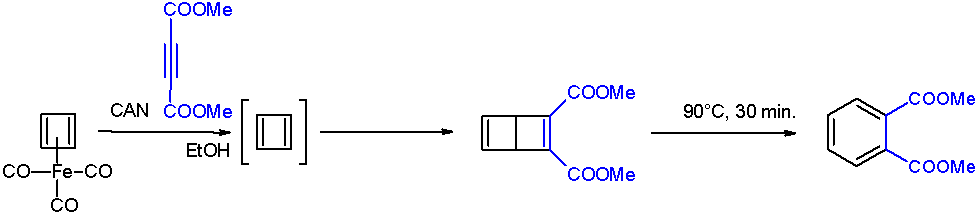
Bicyclobutane
Bicyclo[1.1.0]butane is an organic compound with the formula C4H6. It is a bicyclic molecule consisting of two ''Cis–trans isomerism, cis''-fused cyclopropane rings, and is a colorless and easily condensed gas. Bicyclobutane is noted for being one of the most strain (chemistry), strained compounds that is isolatable on a large scale — its strain energy is estimated at 63.9 kcal mol−1. It is a nonplanar molecule, with a dihedral angle between the two cyclopropane rings of 123°. The first reported bicyclobutane was the ethyl carboxylate derivative, C4H5CO2Et, which was prepared by dehydrohalogenation the corresponding bromocyclobutanecarboxylate ester with sodium hydride. The parent hydrocarbon was prepared from 1-bromo-3-chlorocyclobutane by conversion of the bromocyclobutanecarboxylate ester, followed by intramolecular Wurtz coupling using molten sodium. The intermediate 1-bromo-3-chlorocyclobutane can also be prepared via a modified Hunsdiecker reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octalene
Octalene is a polycyclic hydrocarbon composed of two fused cyclooctatetraene rings. Anions Octalene can be readily reduced by lithium to a dianion and, unusually for such a small molecule, a tetraanion .Müllen, K., Oth, J. F. M., Engels, H.-W. and Vogel, E. (1979), Dianion and Tetraanion Octalene. Angew. Chem. Int. Ed. Engl., 18: 229–231. doi:10.1002/anie.197902291 The di-anion has its two negative charges in one ring, converting that ring into a 10-pi electron aromatic system similar to the di-anion of cyclooctatetraene. In the 18-pi electron tetra-anion, both rings effectively have access to 10 pi electrons, leading to a planar, bicyclic aromatic structure analogous to that of naphthalene. See also *Propalene * Benzyne *Pentalene *Heptalene *Butalene Butalene is a polycyclic hydrocarbon composed of two fused cyclobutadiene rings. A reported possible synthesis of it involves an elimination reaction from a Dewar benzene derivative. The structure itself can be envisione ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually faint, and may be similar to that of gasoline or Naphtha, lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to naturally occurring petroleum, natural gas and coal, or their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are eithe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fused Compound
A bicyclic molecule () is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all of the ring atoms are carbons), or heterocyclic (the rings' atoms consist of at least two elements), like DABCO. Moreover, the two rings can both be aliphatic (''e.g.'' decalin and norbornane), or can be aromatic (''e.g.'' naphthalene), or a combination of aliphatic and aromatic (''e.g.'' tetralin). Three modes of ring junction are possible for a bicyclic compound: * In spiro compounds, the two rings share only one single atom, the spiro atom, which is usually a quaternary carbon. An example of a spirocyclic compound is the photochromic switch spiropyran. * In fused/condensed bicyclic compounds, two rings share two adjacent atoms. In other words, the rings share one covalent bond, ''i.e.'' the bridgehead atoms are directly connected (''e.g.'' α-th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropene
Cyclopropene is an organic compound with the formula . It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental studies of bonding and reactivity. It does not occur naturally, but derivatives are known in some fatty acids. Derivatives of cyclopropene are used commercially to control ripening of some fruit. Structure and bonding The molecule has a triangular structure. The reduced length of the double bond compared to a single bond causes the angle opposite the double bond to narrow to about 51° from the 60° angle found in cyclopropane. As with cyclopropane, the carbon–carbon bonding in the ring has increased p character: the alkene carbon atoms use sp2.68 hybridization for the ring. Synthesis of cyclopropene and derivatives Early syntheses The first confirmed synthesis of cyclopropene, carried out by Dem'yanov and Doyarenko, involved the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Computational Chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of molecules, groups of molecules, and solids. The importance of this subject stems from the fact that, with the exception of some relatively recent findings related to the hydrogen molecular ion (dihydrogen cation), achieving an accurate quantum mechanical depiction of chemical systems analytically, or in a closed form, is not feasible. The complexity inherent in the many-body problem exacerbates the challenge of providing detailed descriptions of quantum mechanical systems. While computational results normally complement information obtained by chemical experiments, it can occasionally predict unobserved chemical phenomena. Overview Computational chemistry differs from theoretical chemistry, which involves a mathematical description of chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parallelogram
In Euclidean geometry, a parallelogram is a simple polygon, simple (non-list of self-intersecting polygons, self-intersecting) quadrilateral with two pairs of Parallel (geometry), parallel sides. The opposite or facing sides of a parallelogram are of equal length and the opposite angles of a parallelogram are of equal measure. The congruence (geometry), congruence of opposite sides and opposite angles is a direct consequence of the Euclidean parallel postulate and neither condition can be proven without appealing to the Euclidean parallel postulate or one of its equivalent formulations. By comparison, a quadrilateral with at least one pair of parallel sides is a trapezoid in American English or a trapezium in British English. The three-dimensional counterpart of a parallelogram is a parallelepiped. The word "parallelogram" comes from the Greek παραλληλό-γραμμον, ''parallēló-grammon'', which means "a shape of parallel lines". Special cases *Rectangle – A par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butadiene
1,3-Butadiene () is the organic compound with the formula CH2=CH-CH=CH2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a material ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure. Structure and reactivity The compound is the prototypical antiaromatic hydrocarbon with 4 pi electrons (or π electrons). It is the smallest 'n''annulene ( annulene). Its rectangular structure is the result of a pseudo- (or second order) Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentalene
Pentalene is a polycyclic hydrocarbon composed of two fused cyclopentadiene rings. It has chemical formula . It is antiaromatic, because it has 4''n'' π electrons where ''n'' is any integer. For this reason it dimerizes even at temperatures as low as −100 °C. The derivative 1,3,5-tri-''tert''-butylpentalene was synthesized in 1973. Because of the ''tert''-butyl substituents this compound is thermally stable. Pentalenes can also be stabilized by benzannulation for example in the compounds benzopentalene and dibenzopentalene. Dilithium pentalenide was isolated in 1962, long before pentalene itself in 1997. It is prepared from reaction of dihydropentalene (pyrolysis of an isomer of dicyclopentadiene) with ''n''-butyllithium in solution and forms a stable salt. In accordance with its structure proton NMR shows 2 signals in a 2 to 1 ratio. The addition of two electrons removes the antiaromaticity; it becomes a planar 10π-electron aromatic species and is thus a bicyclic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heptalene
Heptalene is a polycyclic hydrocarbon with chemical formula , composed of two fused cycloheptatriene rings. It is an unstable, non-planar compound which is non-aromatic. The dianion, however, satisfies Hückel's rule In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π-electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was f ..., is thermally stable, and is planar. See also * Benzocyclooctatetraene References {{Hydrocarbon-stub Polycyclic nonaromatic hydrocarbons Bicyclic compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |