
Computational chemistry is a branch of
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
that uses
computer simulation
Computer simulation is the running of a mathematical model on a computer, the model being designed to represent the behaviour of, or the outcome of, a real-world or physical system. The reliability of some mathematical models can be determin ...
s to assist in solving chemical problems. It uses methods of
theoretical chemistry
Theoretical chemistry is the branch of chemistry which develops theoretical generalizations that are part of the theoretical arsenal of modern chemistry: for example, the concepts of chemical bonding, chemical reaction, valence, the surface ...
incorporated into
computer program
A computer program is a sequence or set of instructions in a programming language for a computer to Execution (computing), execute. It is one component of software, which also includes software documentation, documentation and other intangibl ...
s to calculate the structures and properties of
molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s, groups of molecules, and solids. The importance of this subject stems from the fact that, with the exception of some relatively recent findings related to the hydrogen molecular ion (
dihydrogen cation), achieving an accurate quantum mechanical depiction of chemical systems analytically, or in a closed form, is not feasible. The complexity inherent in the
many-body problem
The many-body problem is a general name for a vast category of physical problems pertaining to the properties of microscopic systems made of many interacting particles. Terminology
''Microscopic'' here implies that quantum mechanics has to be ...
exacerbates the challenge of providing detailed descriptions of quantum mechanical systems. While computational results normally complement information obtained by chemical
experiment
An experiment is a procedure carried out to support or refute a hypothesis, or determine the efficacy or likelihood of something previously untried. Experiments provide insight into cause-and-effect by demonstrating what outcome occurs whe ...
s, it can occasionally predict unobserved chemical
phenomena
A phenomenon ( phenomena), sometimes spelled phaenomenon, is an observable Event (philosophy), event. The term came into its modern Philosophy, philosophical usage through Immanuel Kant, who contrasted it with the noumenon, which ''cannot'' be ...
.
Overview
Computational chemistry differs from
theoretical chemistry
Theoretical chemistry is the branch of chemistry which develops theoretical generalizations that are part of the theoretical arsenal of modern chemistry: for example, the concepts of chemical bonding, chemical reaction, valence, the surface ...
, which involves a mathematical description of chemistry. However, computational chemistry involves the usage of computer programs and additional mathematical skills in order to accurately model various chemical problems. In theoretical chemistry, chemists, physicists, and mathematicians develop
algorithm
In mathematics and computer science, an algorithm () is a finite sequence of Rigour#Mathematics, mathematically rigorous instructions, typically used to solve a class of specific Computational problem, problems or to perform a computation. Algo ...
s and computer programs to predict atomic and molecular properties and reaction paths for
chemical reactions. Computational chemists, in contrast, may simply apply existing computer programs and methodologies to specific chemical questions.
Historically, computational chemistry has had two different aspects:
* Finding a starting point for a laboratory synthesis or assisting in understanding experimental data, such as the position and source of spectroscopic peaks.
* Predicting the possibility of so-far unknown molecules or exploring reaction mechanisms not readily studied via experiments.
As a result, a whole host of algorithms has been put forward by computational chemists.
History
Building on the founding discoveries and theories in the
history of quantum mechanics
The history of quantum mechanics is a fundamental part of the History of physics#20th century: birth of modern physics, history of modern physics. The major chapters of this history begin with the emergence of quantum ideas to explain individual ...
, the first theoretical calculations in chemistry were those of
Walter Heitler
Walter Heinrich Heitler (; 2 January 1904 – 15 November 1981) was a German physicist who made contributions to quantum electrodynamics and quantum field theory. He brought chemistry under quantum mechanics through his theory of valence bondi ...
and
Fritz London
Fritz Wolfgang London (March 7, 1900 – March 30, 1954) was a German born physicist and professor at Duke University. His fundamental contributions to the theories of chemical bonding and of intermolecular forces (London dispersion forces) are to ...
in 1927, using
valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
. The books that were influential in the early development of computational quantum chemistry include
Linus Pauling
Linus Carl Pauling ( ; February 28, 1901August 19, 1994) was an American chemist and peace activist. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics. ''New Scientist'' called him one of the 20 gre ...
and
E. Bright Wilson's 1935 ''Introduction to Quantum Mechanics – with Applications to Chemistry'',
Eyring, Walter and Kimball's 1944 ''Quantum Chemistry'', Heitler's 1945 ''Elementary Wave Mechanics – with Applications to Quantum Chemistry'', and later
Coulson's 1952 textbook ''Valence'', each of which served as primary references for chemists in the decades to follow.
With the development of efficient computer technology in the 1940s, the solutions of elaborate
wave equation
The wave equation is a second-order linear partial differential equation for the description of waves or standing wave fields such as mechanical waves (e.g. water waves, sound waves and seismic waves) or electromagnetic waves (including light ...
s for complex
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
ic systems began to be a realizable objective. In the early 1950s, the first semi-empirical atomic orbital calculations were performed. Theoretical chemists became extensive users of the early digital computers. One significant advancement was marked by Clemens C. J. Roothaan's 1951 paper in the Reviews of Modern Physics.
This paper focused largely on the "LCAO MO" approach (Linear Combination of Atomic Orbitals Molecular Orbitals). For many years, it was the second-most cited paper in that journal.
A very detailed account of such use in the United Kingdom is given by Smith and Sutcliffe. The first ''ab initio''
Hartree–Fock method calculations on diatomic molecules were performed in 1956 at MIT, using a
basis set of
Slater orbitals. For diatomic molecules, a systematic study using a minimum basis set and the first calculation with a larger basis set were published by Ransil and Nesbet respectively in 1960. The first polyatomic calculations using
Gaussian orbitals were performed in the late 1950s. The first
configuration interaction
Configuration interaction (CI) is a post-Hartree–Fock linear variational method for solving the nonrelativistic Schrödinger equation within the Born–Oppenheimer approximation for a quantum chemical multi-electron system. Mathemati ...
calculations were performed in Cambridge on the
EDSAC
The Electronic Delay Storage Automatic Calculator (EDSAC) was an early British computer. Inspired by John von Neumann's seminal ''First Draft of a Report on the EDVAC'', the machine was constructed by Maurice Wilkes and his team at the Universit ...
computer in the 1950s using Gaussian orbitals by
Boys and coworkers. By 1971, when a bibliography of ''ab initio'' calculations was published, the largest molecules included were
naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation ...
and
azulene. Abstracts of many earlier developments in ''ab initio'' theory have been published by Schaefer.
In 1964,
Hückel method calculations (using a simple
linear combination of atomic orbitals
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefunc ...
(LCAO) method to determine electron energies of molecular orbitals of π electrons in conjugated hydrocarbon systems) of molecules, ranging in complexity from
butadiene
1,3-Butadiene () is the organic compound with the formula CH2=CH-CH=CH2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two ...
and
benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
to
ovalene, were generated on computers at Berkeley and Oxford. These empirical methods were replaced in the 1960s by
semi-empirical methods such as
CNDO.
In the early 1970s, efficient ''ab initio'' computer programs such as ATMOL,
Gaussian, IBMOL, and POLYAYTOM, began to be used to speed ''ab initio'' calculations of molecular orbitals.
Of these four programs, only Gaussian, now vastly expanded, is still in use, but many other programs are now in use.
At the same time, the methods of
molecular mechanics
Molecular mechanics uses classical mechanics to model molecular systems. The Born–Oppenheimer approximation is assumed valid and the potential energy of all systems is calculated as a function of the nuclear coordinates using Force field (chemi ...
, such as MM2
force field, were developed, primarily by
Norman Allinger.
One of the first mentions of the term ''computational chemistry'' can be found in the 1970 book ''Computers and Their Role in the Physical Sciences'' by Sidney Fernbach and Abraham Haskell Taub, where they state "It seems, therefore, that 'computational chemistry' can finally be more and more of a reality." During the 1970s, widely different methods began to be seen as part of a new emerging discipline of ''computational chemistry''. The ''
Journal of Computational Chemistry'' was first published in 1980.
Computational chemistry has featured in several Nobel Prize awards, most notably in 1998 and 2013.
Walter Kohn, "for his development of the density-functional theory", and
John Pople
Sir John Anthony Pople (31 October 1925 – 15 March 2004) was a British theoretical chemist who was awarded the Nobel Prize in Chemistry with Walter Kohn in 1998 for his development of computational methods in quantum chemistry.
Ear ...
, "for his development of computational methods in quantum chemistry", received the 1998
Nobel Prize
The Nobel Prizes ( ; ; ) are awards administered by the Nobel Foundation and granted in accordance with the principle of "for the greatest benefit to humankind". The prizes were first awarded in 1901, marking the fifth anniversary of Alfred N ...
in Chemistry.
Martin Karplus,
Michael Levitt and
Arieh Warshel received the 2013
Nobel Prize
The Nobel Prizes ( ; ; ) are awards administered by the Nobel Foundation and granted in accordance with the principle of "for the greatest benefit to humankind". The prizes were first awarded in 1901, marking the fifth anniversary of Alfred N ...
in Chemistry for "the development of multiscale models for complex chemical systems".
Applications
There are several fields within computational chemistry.
* The prediction of the molecular structure of molecules by the use of the simulation of forces, or more accurate quantum chemical methods, to find stationary points on the energy surface as the position of the nuclei is varied.
* Storing and searching for data on chemical entities (see
chemical database A chemical database is a database specifically designed to store chemical information. This information is about chemical and crystal structures, spectra, reactions and syntheses, and thermophysical data.
Types of chemical databases
Bioactiv ...
s).
* Identifying
correlation
In statistics, correlation or dependence is any statistical relationship, whether causal or not, between two random variables or bivariate data. Although in the broadest sense, "correlation" may indicate any type of association, in statistics ...
s between
chemical structure
A chemical structure of a molecule is a spatial arrangement of its atoms and their chemical bonds. Its determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target m ...
s and properties (see ''quantitative structure–property relationship'' (QSPR) and ''
quantitative structure–activity relationship
Quantitative structure–activity relationship models (QSAR models) are regression or classification models used in the chemical and biological sciences and engineering. Like other regression models, QSAR regression models relate a set of "predi ...
'' (QSAR)).
* Computational approaches to help in the efficient synthesis of compounds.
* Computational approaches to design molecules that interact in specific ways with other molecules (e.g.
drug design
Drug design, often referred to as rational drug design or simply rational design, is the invention, inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic compound, organi ...
and
catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
).
These fields can give rise to several applications as shown below.
Catalysis

Computational chemistry is a tool for analyzing catalytic systems without doing experiments. Modern
electronic structure theory and
density functional theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
has allowed researchers to discover and understand
catalysts
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst ...
. Computational studies apply theoretical chemistry to catalysis research. Density functional theory methods calculate the energies and orbitals of molecules to give models of those structures.
Using these methods, researchers can predict values like
activation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
,
site reactivity and other thermodynamic properties.
Data that is difficult to obtain experimentally can be found using computational methods to model the mechanisms of catalytic cycles.
Skilled computational chemists provide predictions that are close to experimental data with proper considerations of methods and basis sets. With good computational data, researchers can predict how catalysts can be improved to lower the cost and increase the efficiency of these reactions.
Drug development
Computational chemistry is used in
drug development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regu ...
to model potentially useful drug molecules and help companies save time and cost in drug development. The drug discovery process involves analyzing data, finding ways to improve current molecules, finding synthetic routes, and testing those molecules.
Computational chemistry helps with this process by giving predictions of which experiments would be best to do without conducting other experiments. Computational methods can also find values that are difficult to find experimentally like
pKa's of compounds. Methods like density functional theory can be used to model drug molecules and find their properties, like their
HOMO and LUMO energies and molecular orbitals. Computational chemists also help companies with developing informatics, infrastructure and designs of drugs.
Aside from drug synthesis,
drug carriers are also researched by computational chemists for
nanomaterials
Nanomaterials describe, in principle, chemical substances or materials of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of nanoscale).
Nanomaterials research takes a materials science ...
. It allows researchers to simulate environments to test the effectiveness and stability of drug carriers. Understanding how water interacts with these nanomaterials ensures stability of the material in human bodies. These computational simulations help researchers optimize the material find the best way to structure these nanomaterials before making them.
Computational chemistry databases
Database
In computing, a database is an organized collection of data or a type of data store based on the use of a database management system (DBMS), the software that interacts with end users, applications, and the database itself to capture and a ...
s are useful for both computational and non computational chemists in research and verifying the validity of computational methods. Empirical data is used to analyze the error of computational methods against experimental data. Empirical data helps researchers with their methods and basis sets to have greater confidence in the researchers results. Computational chemistry databases are also used in testing software or hardware for computational chemistry.
Databases can also use purely calculated data. Purely calculated data uses calculated values over experimental values for databases. Purely calculated data avoids dealing with these adjusting for different experimental conditions like zero-point energy. These calculations can also avoid experimental errors for difficult to test molecules. Though purely calculated data is often not perfect, identifying issues is often easier for calculated data than experimental.
Databases also give public access to information for researchers to use. They contain data that other researchers have found and uploaded to these databases so that anyone can search for them. Researchers use these databases to find information on molecules of interest and learn what can be done with those molecules. Some publicly available chemistry databases include the following.
*
BindingDB: Contains experimental information about protein-small molecule interactions.
*
RCSB: Stores publicly available 3D models of macromolecules (proteins, nucleic acids) and small molecules (drugs, inhibitors)
*
ChEMBL: Contains data from research on drug development such as assay results.
*
DrugBank: Data about mechanisms of drugs can be found here.
Methods
''Ab initio'' method
The programs used in computational chemistry are based on many different
quantum-chemical methods that solve the molecular
Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
associated with the
molecular Hamiltonian
In atomic, molecular, and optical physics and quantum chemistry, the molecular Hamiltonian is the Hamiltonian operator representing the energy of the electrons and nuclei in a molecule. This operator and the associated Schrödinger equation p ...
. Methods that do not include any empirical or semi-empirical parameters in their equations – being derived directly from theory, with no inclusion of experimental data – are called ''
ab initio methods''. A theoretical approximation is rigorously defined on first principles and then solved within an error margin that is qualitatively known beforehand. If numerical iterative methods must be used, the aim is to iterate until full machine accuracy is obtained (the best that is possible with a finite
word length
In computing, a word is any processor design's natural unit of data. A word is a fixed-sized datum handled as a unit by the instruction set or the hardware of the processor. The number of bits or digits in a word (the ''word size'', ''word wid ...
on the computer, and within the mathematical and/or physical approximations made).
Ab initio methods need to define a level of theory (the method) and a
basis set. A basis set consists of functions centered on the molecule's atoms. These sets are then used to describe molecular orbitals via the
linear combination of atomic orbitals
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefunc ...
(LCAO) molecular orbital method
ansatz
In physics and mathematics, an ansatz (; , meaning: "initial placement of a tool at a work piece", plural ansatzes or, from German, ansätze ; ) is an educated guess or an additional assumption made to help solve a problem, and which may later be ...
.

A common type of ''ab initio'' electronic structure calculation is the
Hartree–Fock method (HF), an extension of
molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century. The MOT explains the paramagnetic nature of O2, whic ...
, where electron-electron repulsions in the molecule are not specifically taken into account; only the electrons' average effect is included in the calculation. As the basis set size increases, the energy and wave function tend towards a limit called the Hartree–Fock limit.
Many types of calculations begin with a Hartree–Fock calculation and subsequently correct for electron-electron repulsion, referred to also as
electronic correlation. These types of calculations are termed
post–Hartree–Fock
In computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the s ...
methods. By continually improving these methods, scientists can get increasingly closer to perfectly predicting the behavior of atomic and molecular systems under the framework of quantum mechanics, as defined by the Schrödinger equation. To obtain exact agreement with the experiment, it is necessary to include specific terms, some of which are far more important for heavy atoms than lighter ones.
In most cases, the Hartree–Fock wave function occupies a single configuration or determinant. In some cases, particularly for bond-breaking processes, this is inadequate, and several
configurations must be used.
The total molecular energy can be evaluated as a function of the
molecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that det ...
; in other words, the
potential energy surface
A potential energy surface (PES) or energy landscape describes the energy of a Physical system, system, especially a collection of atoms, in terms of certain Parameter, parameters, normally the positions of the atoms. The Surface (mathematics), ...
. Such a surface can be used for reaction dynamics. The stationary points of the surface lead to predictions of different
isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
s and the
transition structures for conversion between isomers, but these can be determined without full knowledge of the complete surface.

Computational thermochemistry
A particularly important objective, called computational
thermochemistry
Thermochemistry is the study of the heat energy which is associated with chemical reactions and/or phase changes such as melting and boiling. A reaction may release or absorb energy, and a phase change may do the same. Thermochemistry focuses on ...
, is to calculate thermochemical quantities such as the
enthalpy of formation to chemical accuracy. Chemical accuracy is the accuracy required to make realistic chemical predictions and is generally considered to be 1 kcal/mol or 4 kJ/mol. To reach that accuracy in an economic way, it is necessary to use a series of post–Hartree–Fock methods and combine the results. These methods are called
quantum chemistry composite methods
Quantum chemistry composite methods (also referred to as thermochemical recipes) are computational chemistry methods that aim for high accuracy by combining the results of several calculations. They combine methods with a high level of theory and ...
.
Chemical dynamics
After the electronic and
nuclear
Nuclear may refer to:
Physics
Relating to the nucleus of the atom:
*Nuclear engineering
*Nuclear physics
*Nuclear power
*Nuclear reactor
*Nuclear weapon
*Nuclear medicine
*Radiation therapy
*Nuclear warfare
Mathematics
* Nuclear space
*Nuclear ...
variables are
separated within the Born–Oppenheimer representation), the
wave packet
In physics, a wave packet (also known as a wave train or wave group) is a short burst of localized wave action that travels as a unit, outlined by an Envelope (waves), envelope. A wave packet can be analyzed into, or can be synthesized from, a ...
corresponding to the nuclear
degrees of freedom
In many scientific fields, the degrees of freedom of a system is the number of parameters of the system that may vary independently. For example, a point in the plane has two degrees of freedom for translation: its two coordinates; a non-infinite ...
is propagated via the
time evolution operator (physics)
An operator is a function over a space of physical states onto another space of states. The simplest example of the utility of operators is the study of symmetry (which makes the concept of a group useful in this context). Because of this, they a ...
associated to the time-dependent
Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
(for the full
molecular Hamiltonian
In atomic, molecular, and optical physics and quantum chemistry, the molecular Hamiltonian is the Hamiltonian operator representing the energy of the electrons and nuclei in a molecule. This operator and the associated Schrödinger equation p ...
). In the
complementary energy-dependent approach, the time-independent
Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
is solved using the
scattering theory
In physics, scattering is a wide range of physical processes where moving particles or radiation of some form, such as light or sound, are forced to deviate from a straight trajectory by localized non-uniformities (including particles and radiat ...
formalism. The potential representing the interatomic interaction is given by the
potential energy surface
A potential energy surface (PES) or energy landscape describes the energy of a Physical system, system, especially a collection of atoms, in terms of certain Parameter, parameters, normally the positions of the atoms. The Surface (mathematics), ...
s. In general, the
potential energy surface
A potential energy surface (PES) or energy landscape describes the energy of a Physical system, system, especially a collection of atoms, in terms of certain Parameter, parameters, normally the positions of the atoms. The Surface (mathematics), ...
s are coupled via the
vibronic coupling terms.
The most popular methods for propagating the
wave packet
In physics, a wave packet (also known as a wave train or wave group) is a short burst of localized wave action that travels as a unit, outlined by an Envelope (waves), envelope. A wave packet can be analyzed into, or can be synthesized from, a ...
associated to the
molecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that det ...
are:
* the
Chebyshev (real) polynomial,
* the
multi-configuration time-dependent Hartree method (MCTDH),
* the semiclassical method
* and the split operator technique explained below.
= Split operator technique
=
How a computational method solves quantum equations impacts the accuracy and efficiency of the method. The split operator technique is one of these methods for solving differential equations. In computational chemistry, split operator technique reduces computational costs of simulating chemical systems. Computational costs are about how much time it takes for computers to calculate these chemical systems, as it can take days for more complex systems. Quantum systems are difficult and time-consuming to solve for humans. Split operator methods help computers calculate these systems quickly by solving the sub problems in a quantum
differential equation. The method does this by separating the differential equation into two different equations, like when there are more than two operators. Once solved, the split equations are combined into one equation again to give an easily calculable solution.
This method is used in many fields that require solving differential equations, such as
biology
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, History of life, origin, evolution, and ...
. However, the technique comes with a splitting error. For example, with the following solution for a differential equation.
The equation can be split, but the solutions will not be exact, only similar. This is an example of first order splitting.
There are ways to reduce this error, which include taking an average of two split equations.
Another way to increase accuracy is to use higher order splitting. Usually, second order splitting is the most that is done because higher order splitting requires much more time to calculate and is not worth the cost. Higher order methods become too difficult to implement, and are not useful for solving differential equations despite the higher accuracy.
Computational chemists spend much time making systems calculated with split operator technique more accurate while minimizing the computational cost. Calculating methods is a massive challenge for many chemists trying to simulate molecules or chemical environments.
Density functional methods
Density functional theory (DFT) methods are often considered to be ''
ab initio methods'' for determining the molecular electronic structure, even though many of the most common
functionals use parameters derived from empirical data, or from more complex calculations. In DFT, the total energy is expressed in terms of the total one-
electron density
Electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typical ...
rather than the wave function. In this type of calculation, there is an approximate
Hamiltonian
Hamiltonian may refer to:
* Hamiltonian mechanics, a function that represents the total energy of a system
* Hamiltonian (quantum mechanics), an operator corresponding to the total energy of that system
** Dyall Hamiltonian, a modified Hamiltonian ...
and an approximate expression for the total electron density. DFT methods can be very accurate for little computational cost. Some methods combine the density functional exchange functional with the Hartree–Fock exchange term and are termed
hybrid functional methods.
Semi-empirical methods
Semi-empirical
quantum chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions ...
methods are based on the
Hartree–Fock method formalism, but make many approximations and obtain some parameters from empirical data. They were very important in computational chemistry from the 60s to the 90s, especially for treating large molecules where the full Hartree–Fock method without the approximations were too costly. The use of empirical parameters appears to allow some inclusion of correlation effects into the methods.
Primitive semi-empirical methods were designed even before, where the two-electron part of the
Hamiltonian
Hamiltonian may refer to:
* Hamiltonian mechanics, a function that represents the total energy of a system
* Hamiltonian (quantum mechanics), an operator corresponding to the total energy of that system
** Dyall Hamiltonian, a modified Hamiltonian ...
is not explicitly included. For π-electron systems, this was the
Hückel method proposed by
Erich Hückel, and for all valence electron systems, the
extended Hückel method proposed by
Roald Hoffmann
Roald Hoffmann (born Roald Safran; July 18, 1937) is a Polish-American theoretical chemist who won the 1981 Nobel Prize in Chemistry. He has also published plays and poetry. He is the Frank H. T. Rhodes Professor of Humane Letters Emeritus at C ...
. Sometimes, Hückel methods are referred to as "completely empirical" because they do not derive from a Hamiltonian. Yet, the term "empirical methods", or "empirical force fields" is usually used to describe molecular mechanics.
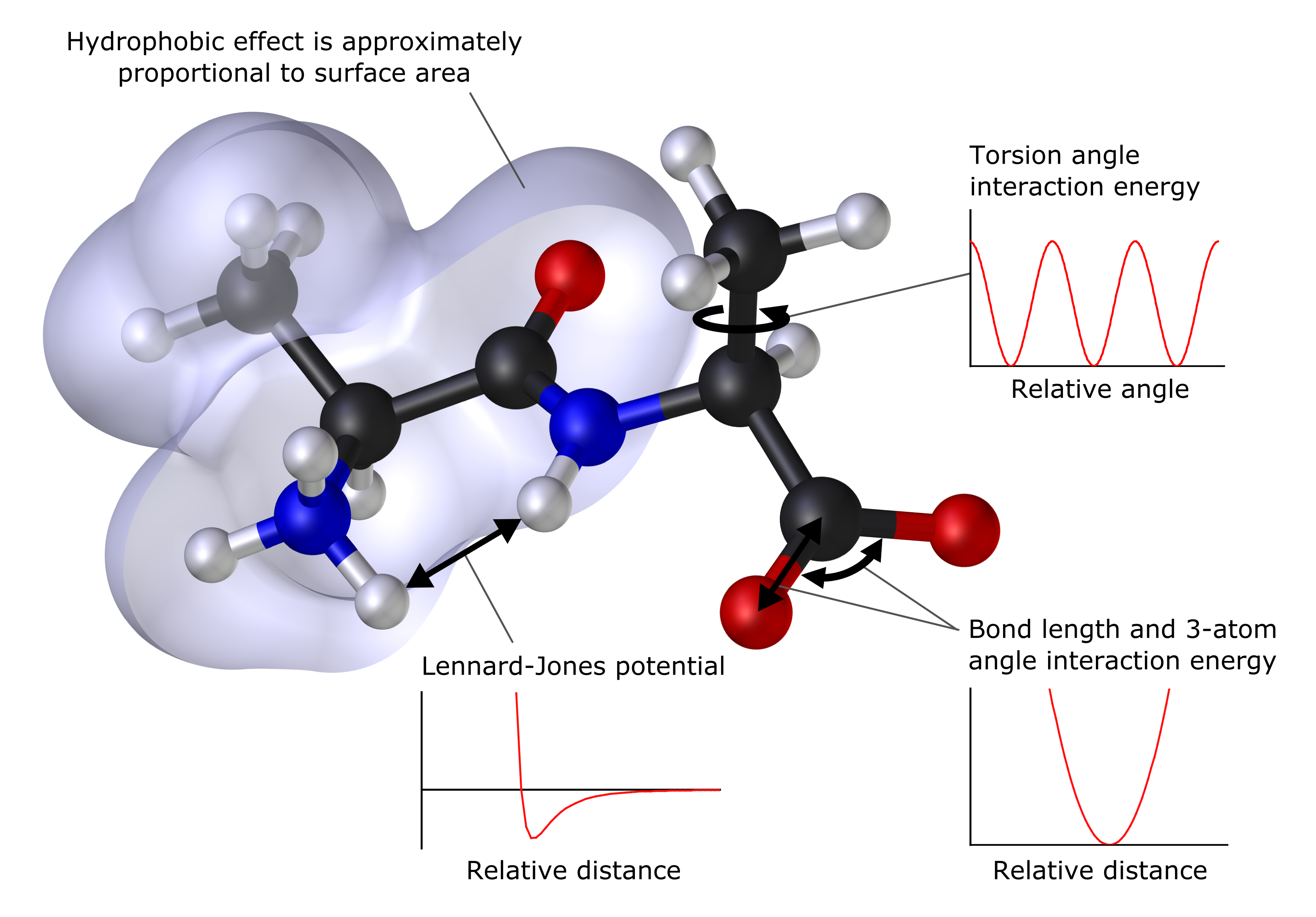
Molecular mechanics
In many cases, large molecular systems can be modeled successfully while avoiding quantum mechanical calculations entirely.
Molecular mechanics
Molecular mechanics uses classical mechanics to model molecular systems. The Born–Oppenheimer approximation is assumed valid and the potential energy of all systems is calculated as a function of the nuclear coordinates using Force field (chemi ...
simulations, for example, use one classical expression for the energy of a compound, for instance, the
harmonic oscillator
In classical mechanics, a harmonic oscillator is a system that, when displaced from its equilibrium position, experiences a restoring force ''F'' proportional to the displacement ''x'':
\vec F = -k \vec x,
where ''k'' is a positive const ...
. All constants appearing in the equations must be obtained beforehand from experimental data or ''ab initio'' calculations.
The database of compounds used for parameterization, i.e. the resulting set of parameters and functions is called the
force field, is crucial to the success of molecular mechanics calculations. A force field parameterized against a specific class of molecules, for instance, proteins, would be expected to only have any relevance when describing other molecules of the same class.
These methods can be applied to proteins and other large biological molecules, and allow studies of the approach and interaction (docking) of potential drug molecules.
Molecular dynamics
Molecular dynamics (MD) use either
quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
,
molecular mechanics
Molecular mechanics uses classical mechanics to model molecular systems. The Born–Oppenheimer approximation is assumed valid and the potential energy of all systems is calculated as a function of the nuclear coordinates using Force field (chemi ...
or a
mixture of both to calculate forces which are then used to solve
Newton's laws of motion
Newton's laws of motion are three physical laws that describe the relationship between the motion of an object and the forces acting on it. These laws, which provide the basis for Newtonian mechanics, can be paraphrased as follows:
# A body re ...
to examine the time-dependent behavior of systems. The result of a molecular dynamics simulation is a trajectory that describes how the position and velocity of particles varies with time. The phase point of a system described by the positions and momenta of all its particles on a previous time point will determine the next phase point in time by integrating over Newton's laws of motion.
Monte Carlo
Monte Carlo
Monte Carlo ( ; ; or colloquially ; , ; ) is an official administrative area of Monaco, specifically the Ward (country subdivision), ward of Monte Carlo/Spélugues, where the Monte Carlo Casino is located. Informally, the name also refers to ...
(MC) generates configurations of a system by making random changes to the positions of its particles, together with their orientations and conformations where appropriate. It is a random sampling method, which makes use of the so-called ''importance sampling''. Importance sampling methods are able to generate low energy states, as this enables properties to be calculated accurately. The potential energy of each configuration of the system can be calculated, together with the values of other properties, from the positions of the atoms.
Quantum mechanics/molecular mechanics (QM/MM)
QM/MM is a hybrid method that attempts to combine the accuracy of quantum mechanics with the speed of molecular mechanics. It is useful for simulating very large molecules such as
enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
s.
Quantum Computational Chemistry
Quantum computational chemistry
Quantum computational chemistry is an emerging field that exploits quantum computing to simulate chemical systems. Despite quantum mechanics' foundational role in understanding chemical behaviors, traditional computational approaches face signi ...
aims to exploit
quantum computing
A quantum computer is a computer that exploits quantum mechanical phenomena. On small scales, physical matter exhibits properties of wave-particle duality, both particles and waves, and quantum computing takes advantage of this behavior using s ...
to simulate chemical systems, distinguishing itself from the QM/MM (Quantum Mechanics/Molecular Mechanics) approach. While QM/MM uses a hybrid approach, combining quantum mechanics for a portion of the system with classical mechanics for the remainder, quantum computational chemistry exclusively uses quantum computing methods to represent and process information, such as Hamiltonian operators.
Conventional computational chemistry methods often struggle with the complex quantum mechanical equations, particularly due to the exponential growth of a quantum system's wave function. Quantum computational chemistry addresses these challenges using
quantum computing methods, such as qubitization and
quantum phase estimation, which are believed to offer scalable solutions.
Qubitization involves adapting the Hamiltonian operator for more efficient processing on quantum computers, enhancing the simulation's efficiency. Quantum phase estimation, on the other hand, assists in accurately determining energy eigenstates, which are critical for understanding the quantum system's behavior.
While these techniques have advanced the field of computational chemistry, especially in the simulation of chemical systems, their practical application is currently limited mainly to smaller systems due to technological constraints. Nevertheless, these developments may lead to significant progress towards achieving more precise and resource-efficient quantum chemistry simulations.
Computational costs in chemistry algorithms
The computational cost and algorithmic complexity in chemistry are used to help understand and predict chemical phenomena. They help determine which algorithms/computational methods to use when solving chemical problems. This section focuses on the scaling of computational complexity with molecule size and details the algorithms commonly used in both domains.
In quantum chemistry, particularly, the complexity can grow exponentially with the number of electrons involved in the system. This exponential growth is a significant barrier to simulating large or complex systems accurately.
Advanced algorithms in both fields strive to balance accuracy with computational efficiency. For instance, in MD, methods like
Verlet integration
Verlet integration () is a numerical method used to integrate Newton's equations of motion. It is frequently used to calculate trajectories of particles in molecular dynamics simulations and computer graphics. The algorithm was first used in 17 ...
or
Beeman's algorithm are employed for their computational efficiency. In quantum chemistry, hybrid methods combining different computational approaches (like QM/MM) are increasingly used to tackle large biomolecular systems.
Algorithmic complexity examples
The following list illustrates the impact of computational complexity on algorithms used in chemical computations. It is important to note that while this list provides key examples, it is not comprehensive and serves as a guide to understanding how computational demands influence the selection of specific computational methods in chemistry.
Molecular dynamics
Algorithm
Solves Newton's equations of motion for atoms and molecules.
Complexity
The standard pairwise interaction calculation in MD leads to an
complexity for
particles. This is because each particle interacts with every other particle, resulting in
interactions. Advanced algorithms, such as the Ewald summation or Fast Multipole Method, reduce this to
or even
by grouping distant particles and treating them as a single entity or using clever mathematical approximations.
Quantum mechanics/molecular mechanics (QM/MM)
Algorithm
Combines quantum mechanical calculations for a small region with molecular mechanics for the larger environment.
Complexity
The complexity of QM/MM methods depends on both the size of the quantum region and the method used for quantum calculations. For example, if a Hartree-Fock method is used for the quantum part, the complexity can be approximated as
, where
is the number of basis functions in the quantum region. This complexity arises from the need to solve a set of coupled equations iteratively until self-consistency is achieved.

Hartree-Fock method
Algorithm
Finds a single Fock state that minimizes the energy.
Complexity
NP-hard or NP-complete as demonstrated by embedding instances of the
Ising model
The Ising model (or Lenz–Ising model), named after the physicists Ernst Ising and Wilhelm Lenz, is a mathematical models in physics, mathematical model of ferromagnetism in statistical mechanics. The model consists of discrete variables that r ...
into Hartree-Fock calculations. The Hartree-Fock method involves solving the Roothaan-Hall equations, which scales as
to
depending on implementation, with
being the number of basis functions. The computational cost mainly comes from evaluating and transforming the two-electron integrals. This proof of NP-hardness or NP-completeness comes from embedding problems like the Ising model into the Hartree-Fock formalism.

Density functional theory
Algorithm
Investigates the
electronic structure
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions ...
or
nuclear structure
Understanding the structure of the atomic nucleus is one of the central challenges in nuclear physics.
Models The cluster model
The cluster model describes the nucleus as a molecule-like collection of proton-neutron groups (e.g., alpha particl ...
of
many-body systems such as atoms, molecules, and the
condensed phases.
Complexity
Traditional implementations of DFT typically scale as
, mainly due to the need to diagonalize the
Kohn-Sham matrix. The diagonalization step, which finds the eigenvalues and eigenvectors of the matrix, contributes most to this scaling. Recent advances in DFT aim to reduce this complexity through various approximations and algorithmic improvements.
Standard CCSD and CCSD(T) method
Algorithm
CCSD and CCSD(T) methods are advanced electronic structure techniques involving single, double, and in the case of CCSD(T), perturbative triple excitations for calculating electronic correlation effects.
Complexity
= CCSD
=
Scales as
where
is the number of basis functions. This intense computational demand arises from the inclusion of single and double excitations in the electron correlation calculation.
= CCSD(T)
=
With the addition of perturbative triples, the complexity increases to
. This elevated complexity restricts practical usage to smaller systems, typically up to 20-25 atoms in conventional implementations.

Linear-scaling CCSD(T) method
Algorithm
An adaptation of the standard CCSD(T) method using local natural orbitals (NOs) to significantly reduce the computational burden and enable application to larger systems.
Complexity
Achieves linear scaling with the system size, a major improvement over the traditional fifth-power scaling of CCSD. This advancement allows for practical applications to molecules of up to 100 atoms with reasonable basis sets, marking a significant step forward in computational chemistry's capability to handle larger systems with high accuracy.
Proving the complexity classes for algorithms involves a combination of mathematical proof and computational experiments. For example, in the case of the Hartree-Fock method, the proof of NP-hardness is a theoretical result derived from complexity theory, specifically through reductions from known
NP-hard
In computational complexity theory, a computational problem ''H'' is called NP-hard if, for every problem ''L'' which can be solved in non-deterministic polynomial-time, there is a polynomial-time reduction from ''L'' to ''H''. That is, assumi ...
problems.
For other methods like MD or DFT, the computational complexity is often empirically observed and supported by algorithm analysis. In these cases, the proof of correctness is less about formal mathematical proofs and more about consistently observing the computational behaviour across various systems and implementations.
Accuracy
Computational chemistry is not an ''exact'' description of real-life chemistry, as the mathematical and physical models of nature can only provide an approximation. However, the majority of chemical phenomena can be described to a certain degree in a qualitative or approximate quantitative computational scheme.
Molecules consist of nuclei and electrons, so the methods of
quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
apply. Computational chemists often attempt to solve the non-relativistic
Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
, with relativistic corrections added, although some progress has been made in solving the fully relativistic
Dirac equation
In particle physics, the Dirac equation is a relativistic wave equation derived by British physicist Paul Dirac in 1928. In its free form, or including electromagnetic interactions, it describes all spin-1/2 massive particles, called "Dirac ...
. In principle, it is possible to solve the Schrödinger equation in either its time-dependent or time-independent form, as appropriate for the problem in hand; in practice, this is not possible except for very small systems. Therefore, a great number of approximate methods strive to achieve the best trade-off between accuracy and computational cost.
Accuracy can always be improved with greater computational cost. Significant errors can present themselves in
ab initio
( ) is a Latin term meaning "from the beginning" and is derived from the Latin ("from") + , ablative singular of ("beginning").
Etymology
, from Latin, literally "from the beginning", from ablative case of "entrance", "beginning", related t ...
models comprising many electrons, due to the computational cost of full relativistic-inclusive methods.
This complicates the study of molecules interacting with high atomic mass unit atoms, such as transitional metals and their catalytic properties. Present algorithms in computational chemistry can routinely calculate the properties of small molecules that contain up to about 40 electrons with errors for energies less than a few kJ/mol. For geometries, bond lengths can be predicted within a few picometers and bond angles within 0.5 degrees. The treatment of larger molecules that contain a few dozen atoms is computationally tractable by more approximate methods such as
density functional theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
(DFT).
There is some dispute within the field whether or not the latter methods are sufficient to describe complex chemical reactions, such as those in biochemistry. Large molecules can be studied by semi-empirical approximate methods. Even larger molecules are treated by
classical mechanics
Classical mechanics is a Theoretical physics, physical theory describing the motion of objects such as projectiles, parts of Machine (mechanical), machinery, spacecraft, planets, stars, and galaxies. The development of classical mechanics inv ...
methods that use what are called
molecular mechanics
Molecular mechanics uses classical mechanics to model molecular systems. The Born–Oppenheimer approximation is assumed valid and the potential energy of all systems is calculated as a function of the nuclear coordinates using Force field (chemi ...
(MM).In QM-MM methods, small parts of large complexes are treated quantum mechanically (QM), and the remainder is treated approximately (MM).
Software packages
Many self-sufficient
computational chemistry software packages exist. Some include many methods covering a wide range, while others concentrate on a very specific range or even on one method. Details of most of them can be found in:
*
Biomolecular modelling programs:
proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, re ...
,
nucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
.
*
Molecular mechanics
Molecular mechanics uses classical mechanics to model molecular systems. The Born–Oppenheimer approximation is assumed valid and the potential energy of all systems is calculated as a function of the nuclear coordinates using Force field (chemi ...
programs.
*
Quantum chemistry and solid state-physics software supporting several methods.
*
Molecular design software Molecular design software is notable software for molecular modeling, that provides special support for developing molecular models ''de novo''.
In contrast to the usual molecular modeling programs, such as for molecular dynamics and quantum chemi ...
*
Semi-empirical programs.
*
Valence bond programs.
Specialized journals on computational chemistry
*
Annual Reports in Computational Chemistry'
* ''
Computational and Theoretical Chemistry''
*
Computational and Theoretical Polymer Science'
*
Computers & Chemical Engineering
* ''
Journal of Chemical Information and Modeling
The ''Journal of Chemical Information and Modeling'' is a peer-reviewed scientific journal published by the American Chemical Society. It was established in 1961 as the ''Journal of Chemical Documentation'', renamed in 1975 to ''Journal of Chemical ...
''
*
Journal of Chemical Software'
* ''
Journal of Chemical Theory and Computation''
* ''
Journal of Cheminformatics
The ''Journal of Cheminformatics'' is a peer-reviewed open access scientific journal that covers cheminformatics and molecular modelling. It was established in 2009 with David Wild (Indiana University) and Christoph Steinbeck (then at EMBL-EBI) as ...
''
* ''
Journal of Computational Chemistry''
*
Journal of Computer Aided Chemistry'
*
Journal of Computer Chemistry Japan'
*
Journal of Computer-aided Molecular Design'
* ''
Journal of Theoretical and Computational Chemistry''
* ''
Molecular Informatics''
* ''
Theoretical Chemistry Accounts''
External links
NIST Computational Chemistry Comparison and Benchmark DataBase– Contains a database of thousands of computational and experimental results for hundreds of systems
American Chemical Society Division of Computers in Chemistry– American Chemical Society Computers in Chemistry Division, resources for grants, awards, contacts and meetings.
CSTB reportMathematical Research in Materials Science: Opportunities and Perspectives – CSTB Report
3.320 Atomistic Computer Modeling of Materials (SMA 5107)Free
MIT
The Massachusetts Institute of Technology (MIT) is a private research university in Cambridge, Massachusetts, United States. Established in 1861, MIT has played a significant role in the development of many areas of modern technology and sc ...
Course
Chem 4021/8021 Computational ChemistryFree
University of Minnesota
The University of Minnesota Twin Cities (historically known as University of Minnesota) is a public university, public Land-grant university, land-grant research university in the Minneapolis–Saint Paul, Twin Cities of Minneapolis and Saint ...
Course
Technology Roadmap for Computational ChemistryApplications of molecular and materials modelling.Impact of Advances in Computing and Communications Technologies on Chemical Science and Technology CSTB ReportSusi Lehtola, Antti J. Karttunen:"Free and open source software for computational chemistry education", First published: 23 March 2022, https://doi.org/10.1002/wcms.1610 (Open Access)
CCL.NET: Computational Chemistry List, Ltd.
See also
References
{{Authority control
Computational fields of study
Theoretical chemistry
Physical chemistry
Chemical physics
Computational physics
 Computational chemistry is a branch of
Computational chemistry is a branch of  Computational chemistry is a tool for analyzing catalytic systems without doing experiments. Modern electronic structure theory and
Computational chemistry is a tool for analyzing catalytic systems without doing experiments. Modern electronic structure theory and 



