|
Phyrrotite
Pyrrhotite ('' pyrrhos'' in Greek meaning "flame-coloured"'')'' is an iron sulfide mineral with the formula Fe(1−x)S (x = 0 to 0.125). It is a nonstoichiometric variant of FeS, the mineral known as troilite. Pyrrhotite is also called magnetic pyrite, because the color is similar to pyrite and it is weakly magnetic. The magnetism decreases as the iron content increases, and troilite is non-magnetic. Pyrrhotite is generally tabular and brassy/bronze in color with a metallic luster. The mineral occurs with mafic igneous rocks like norites, and may form from pyrite during metamorphic processes. Pyrrhotite is associated and mined with other sulfide minerals like pentlandite, pyrite, chalcopyrite, and magnetite, and has been found globally. Structure Pyrrhotite exists as a number of polytypes of hexagonal or monoclinic crystal symmetry; several polytypes often occur within the same specimen. Their structure is based on the NiAs unit cell. As such, Fe occupies an octahedra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sphalerite
Sphalerite is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in Sedimentary exhalative deposits, sedimentary exhalative, Carbonate-hosted lead-zinc ore deposits, Mississippi-Valley type, and Volcanogenic massive sulfide ore deposit, volcanogenic massive sulfide deposits. It is found in association with galena, chalcopyrite, pyrite (and other sulfide mineral, sulfides), calcite, dolomite (mineral), dolomite, quartz, rhodochrosite, and fluorite. German geologist Ernst Friedrich Glocker discovered sphalerite in 1847, naming it based on the Greek word ''sphaleros'', meaning "deceiving", due to the difficulty of identifying the mineral. In addition to zinc, sphalerite is an ore of cadmium, gallium, germanium, and indium. Miners have been known to refer to sphalerite as ''zinc blende'', ''black-jack'', and ''ruby blende''. Marmatite is an opaque black variety with a high iron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentlandite
Pentlandite is an iron–nickel sulfide with the chemical formula . Pentlandite has a narrow variation range in nickel to iron ratios (Ni:Fe), but it is usually described as 1:1. In some cases, this ratio is skewed by the presence of pyrrhotite Inclusion (mineral), inclusions. It also contains minor cobalt, usually at low levels as a fraction of weight. Pentlandite forms Cubic (crystal system), isometric crystals, but it is normally found in massive granular Aggregate (geology), aggregates. It is brittle with a Mohs scale of mineral hardness, hardness of 3.5–4 and specific gravity of 4.6–5.0 and is non-magnetic. It has a yellowish bronze color and a metallic Lustre (mineralogy), luster. Pentlandite is found in abundance within Ultramafic rock, ultramafic rocks, making it one of the most important sources of mined nickel. It also occasionally occurs within mantle xenoliths and "black smoker" hydrothermal vents. Etymology It is named after Republic of Ireland, Irish scientist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trigonal Prismatic Molecular Geometry
In chemistry, the trigonal prismatic molecular geometry describes the shape of compounds where six atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a triangular prism. The structure commonly occurs for d0, d1 and d2 transition metal complexes with covalently-bound ligands and small charge separation. In d0 complexes it may be ascribed to sd5 hybridization, but in d1 and d2 complexes the d''z''2 orbital is occupied by nonbonding electron (pair). Furthermore, when unoccupied, said orbital participates in bonding and causes C3v distortion, like in W(CH3)6. Examples Hexamethyltungsten (W(CH3)6) was the first example of a molecular trigonal prismatic complex. The figure shows the six carbon atoms arranged at the vertices of a triangular prism with the tungsten at the centre. The hydrogen atoms are not shown. Some other transition metals have trigonal prismatic hexamethyl complexes, including both neutral molecules such as Mo(CH3)6 and Re( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Coordination Geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix '' octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The concept of octahedral coordination geometry was developed by Alfred Wern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
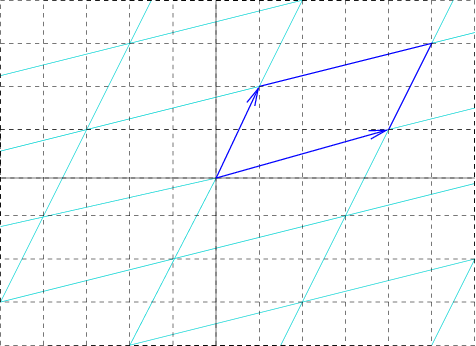
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector In mathematics, a unit vector in a normed vector space is a Vector (mathematics and physics), vector (often a vector (geometry), spatial vector) of Norm (mathematics), length 1. A unit vector is often denoted by a lowercase letter with a circumfle ..., for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
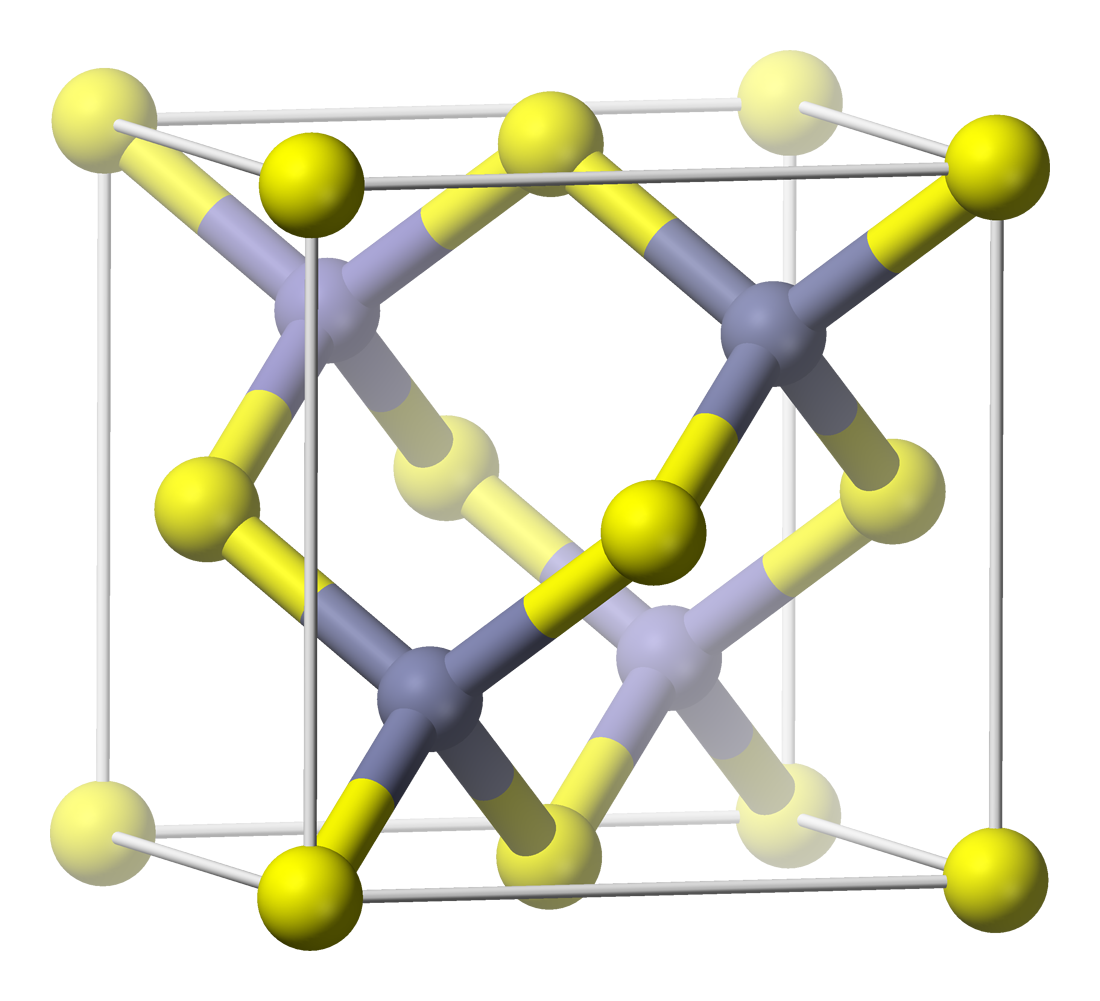
Nickel Arsenide
Nickel arsenide is a compound of nickel and arsenic and component of the ore nickeline. It is highly toxic and a known carcinogen in humans. Uncontrolled decomposition of nickel arsenide can give rise to further toxic nickel compounds. Toxicity Nickel arsenide was one of the first compounds that revealed the toxicity of nickel. The damage to the miners' lungs was documented by Georgius Agricola in the 16th century: "kupfer-nickel" ores in the Schneeberg mines contained red-colored NiAs mineral originally mistaken for the copper ore, thus the (copper) in the name. The (demon) name was reflecting the damage it did to the health of the workers, in addition to them being unable to extract any copper from this ore. The acute oral LD50 In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal dose of a given substance. The value of LD50 for a substance is the dose req ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Symmetry
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter. The smallest group of particles in a material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of principal axes/edges, of the unit cell and angles between them are lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of a crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three Vector (geometric), vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic system. They form a parallelogram prism (geometry), prism. Hence two pairs of vectors are perpendicular (meet at right angles), while the third pair makes an angle other than 90°. Bravais lattices Two monoclinic Bravais lattices exist: the primitive monoclinic and the base-centered monoclinic. For the base-centered monoclinic lattice, the primitive cell has the shape of an oblique rhombic prism;See , row mC, column Primitive, where the cell parameters are given as a1 = a2, α = β it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. The length a of the primitive cell below equals \frac \sqrt of the conventional cell above. Crystal class ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexagonal Crystal System
In crystallography, the hexagonal crystal family is one of the six crystal family, crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section hexagonal crystal family#Crystal systems, crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polytypes
In crystallography, polymorphism is the phenomenon where a compound or element can crystallize into more than one crystal structure. The preceding definition has evolved over many years and is still under discussion today. Discussion of the defining characteristics of polymorphism involves distinguishing among types of transitions and structural changes occurring in polymorphism versus those in other phenomena. Overview Phase transitions (phase changes) that help describe polymorphism include polymorphic transitions as well as melting and vaporization transitions. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." Additionally, Walter McCrone described the phases in polymorphic matter as "different in crystal structure but identical in the liquid or vapor states." McCrone also define ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrhotite (Polarized Light)
Pyrrhotite ('' pyrrhos'' in Greek meaning "flame-coloured"'')'' is an iron sulfide mineral with the formula Fe(1−x)S (x = 0 to 0.125). It is a nonstoichiometric variant of FeS, the mineral known as troilite. Pyrrhotite is also called magnetic pyrite, because the color is similar to pyrite and it is weakly magnetic. The magnetism decreases as the iron content increases, and troilite is non-magnetic. Pyrrhotite is generally tabular and brassy/bronze in color with a metallic luster. The mineral occurs with mafic igneous rocks like norites, and may form from pyrite during metamorphic processes. Pyrrhotite is associated and mined with other sulfide minerals like pentlandite, pyrite, chalcopyrite, and magnetite, and has been found globally. Structure Pyrrhotite exists as a number of polytypes of hexagonal or monoclinic crystal symmetry; several polytypes often occur within the same specimen. Their structure is based on the NiAs unit cell. As such, Fe occupies an octahedra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |