|
Photostability
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible (400–750 nm), or infrared radiation (750–2500 nm). In nature, photochemistry is of immense importance as it is the basis of photosynthesis, vision, and the formation of vitamin D with sunlight. It is also responsible for the appearance of DNA mutations leading to skin cancers. Photochemical reactions proceed differently than temperature-driven reactions. Photochemical paths access high-energy intermediates that cannot be generated thermally, thereby overcoming large activation barriers in a short period of time, and allowing reactions otherwise inaccessible by thermal processes. Photochemistry can also be destructive, as illustrated by the photodegradation of plastics. Concept Grotthuss–Draper law and Stark–Einstein law Photoexci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Johannes Stark
Johannes Stark (; 15 April 1874 – 21 June 1957) was a German physicist who received the Nobel Prize in Physics in 1919 "for his discovery of the Doppler effect in canal rays and the splitting of spectral lines in electric fields". This phenomenon is known as the Stark effect. Stark received his Ph.D. in physics from the University of Munich in 1897 under the supervision of Eugen von Lommel, and served as Lommel's assistant until his appointment as a lecturer at the University of Göttingen in 1900. He was an extraordinary professor at Leibniz University Hannover from 1906 until he became a professor at RWTH Aachen University in 1909. In 1917, he became professor at the University of Greifswald, and he also worked at the University of Würzburg from 1920 to 1922. A supporter of Adolf Hitler from 1924, Stark was one of the main figures, along with fellow Nobel laureate Philipp Lenard, in the antisemitic '' Deutsche Physik'' movement, which sought to remove Jewish scientists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
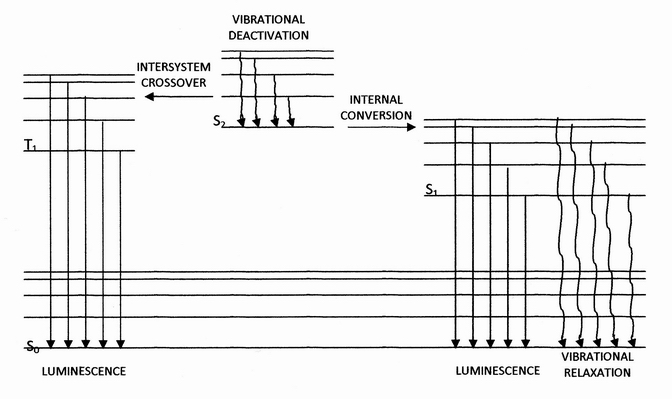
Triplet State
In quantum mechanics, a triplet state, or spin triplet, is the quantum state of an object such as an electron, atom, or molecule, having a quantum spin ''S'' = 1. It has three allowed values of the spin's projection along a given axis ''m''S = −1, 0, or +1, giving the name "triplet". Spin, in the context of quantum mechanics, is not a mechanical rotation but a more abstract concept that characterizes a particle's intrinsic angular momentum. It is particularly important for systems at atomic length scales, such as individual atoms, protons, or electrons. A triplet state occurs in cases where the spins of two unpaired electrons, each having spin ''s'' = , align to give ''S'' = 1, in contrast to the more common case of two electrons aligning oppositely to give ''S'' = 0, a spin singlet. Most molecules encountered in daily life exist in a singlet state because all of their electrons are paired, but molecular oxygen is an exception. At room temperature, O2 exists in a triplet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colored visible light. The color of the light emitted depends on the chemical composition of the substance. Fluorescent materials generally cease to glow nearly immediately when the radiation source stops. This distinguishes them from the other type of light emission, phosphorescence. Phosphorescent materials continue to emit light for some time after the radiation stops. This difference in duration is a result of quantum spin effects. Fluorescence occurs when a photon from incoming radiation is absorbed by a molecule, exciting it to a higher energy level, followed by the emission of light as the molecule returns to a lower energy state. The emitted light may have a longer wavelength and, therefore, a lower photon energy than the absorbed radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Conversion (chemistry)
Internal conversion is a transition from a higher to a lower electronic state in a molecule or atom.A general and quantitative discussion of intramolecular radiationless transitions is the subject of an article by M. Bixon and J. Jortner (''J. Chem. Phys.'', 48 (2) 715-726 (1968)). It is sometimes called "radiationless de-excitation", because no photons are emitted. It differs from intersystem crossing in that, while both are radiationless methods of de-excitation, the molecular spin state for internal conversion remains the same, whereas it changes for intersystem crossing. The energy of the electronically excited state is given off to vibrational modes of the molecule. The excitation energy is transformed into heat. Examples A classic example of this process is the quinine sulfate fluorescence, which can be quenched by the use of various halide salts. The excited molecule can de-excite by increasing the thermal energy of the surrounding solvated ions. Several natural molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kasha's Rule
Kasha's rule is a principle in the photochemistry of electronically excited molecules. The rule states that photon emission (fluorescence or phosphorescence) occurs in appreciable yield only from the lowest excited state of a given multiplicity. It is named after American spectroscopist Michael Kasha, who proposed it in 1950. Description and explanation The rule is relevant in understanding the emission spectrum of an excited molecule. Upon absorbing a photon, a molecule in its electronic ground state (denoted ''S''0, assuming a singlet state) may – depending on the photon wavelength – be excited to any of a set of higher electronic states (denoted ''S''n where ''n''>0). However, according to Kasha's rule, photon emission (termed fluorescence in the case of an ''S'' state) is expected in appreciable yield only from the lowest excited state, ''S''1. Since only one state is expected to yield emission, an equivalent statement of the rule is that the emission wavelength is in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontier orbitals'', such as in the frontier molecular orbital theory. Gap The energy difference between the HOMO and LUMO is ''the HOMO–LUMO gap''. Its size can be used to predict the strength and stability of transition metal Coordination complex, complexes, as well as the colors they produce in solution. As a rule of thumb, the smaller a compound's HOMO–LUMO gap, the less stable the compound. Recent quantum‐chemical analyses of over 700 compounds demonstrated that terrestrial secondary metabolites exhibit HOMO–LUMO gaps on average about 2 eV narrower than organic molecules found in carbonaceous meteorites, and that combining gap width with hydrophilicity creates a robust discriminator between biotic and abiotic chemistries. This sugges ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HOMO
''Homo'' () is a genus of great ape (family Hominidae) that emerged from the genus ''Australopithecus'' and encompasses only a single extant species, ''Homo sapiens'' (modern humans), along with a number of extinct species (collectively called archaic humans) classified as either ancestral or closely related to modern humans; these include ''Homo erectus'' and ''Homo neanderthalensis''. The oldest member of the genus is ''Homo habilis'', with records of just over 2 million years ago. ''Homo'', together with the genus ''Paranthropus'', is probably most closely related to the species ''Australopithecus africanus'' within ''Australopithecus''.'''' The closest living relatives of ''Homo'' are of the genus ''Pan (genus), Pan'' (chimpanzees and bonobos), with the ancestors of ''Pan'' and ''Homo'' estimated to have diverged around 5.7–11 million years ago during the Late Miocene. ''H. erectus'' appeared about 2 million years ago and spread throughout Africa (deba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Singlet State
In quantum mechanics, a singlet state usually refers to a system in which all electrons are paired. The term 'singlet' originally meant a linked set of particles whose net angular momentum is zero, that is, whose overall spin quantum number s=0. As a result, there is only one spectral line of a singlet state. In contrast, a doublet state contains one unpaired electron and shows splitting of spectral lines into a doublet, and a triplet state has two unpaired electrons and shows threefold splitting of spectral lines. History Singlets and the related Spin (physics), spin concepts of Doublet state, doublets and Triplet state, triplets occur frequently in atomic physics and nuclear physics, where one often needs to determine the total spin of a collection of particles. Since the only observed fundamental particle with zero spin is the extremely inaccessible Higgs boson, singlets in everyday physics are necessarily composed of sets of particles whose individual spins are non-zero, e.g. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conservation Of Angular Momentum
Angular momentum (sometimes called moment of momentum or rotational momentum) is the rotational analog of Momentum, linear momentum. It is an important physical quantity because it is a Conservation law, conserved quantity – the total angular momentum of a closed system remains constant. Angular momentum has both a direction (geometry), direction and a magnitude, and both are conserved. Bicycle and motorcycle dynamics, Bicycles and motorcycles, flying discs, Rifling, rifled bullets, and gyroscopes owe their useful properties to conservation of angular momentum. Conservation of angular momentum is also why hurricanes form spirals and neutron stars have high rotational rates. In general, conservation limits the possible motion of a system, but it does not uniquely determine it. The three-dimensional angular momentum for a point particle is classically represented as a pseudovector , the cross product of the particle's position vector (relative to some origin) and its mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin (physics)
Spin is an Intrinsic and extrinsic properties, intrinsic form of angular momentum carried by elementary particles, and thus by List of particles#Composite particles, composite particles such as hadrons, atomic nucleus, atomic nuclei, and atoms. Spin is quantized, and accurate models for the interaction with spin require relativistic quantum mechanics or quantum field theory. The existence of electron spin angular momentum is inferred from experiments, such as the Stern–Gerlach experiment, in which silver atoms were observed to possess two possible discrete angular momenta despite having no orbital angular momentum. The relativistic spin–statistics theorem connects electron spin quantization to the Pauli exclusion principle: observations of exclusion imply half-integer spin, and observations of half-integer spin imply exclusion. Spin is described mathematically as a vector for some particles such as photons, and as a spinor or bispinor for other particles such as electrons. Sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |