|
P-site
The P-site (for peptidyl) is the second binding site for tRNA in the ribosome. The other two sites are the A-site (aminoacyl), which is the first binding site in the ribosome, and the E-site (exit), the third. During protein translation, the P-site holds the tRNA which is linked to the growing polypeptide chain. When a stop codon is reached, the peptidyl-tRNA bond of the tRNA located in the P-site is cleaved releasing the newly synthesized protein. During the translocation step of the elongation phase, the mRNA is advanced by one codon, coupled to movement of the tRNAs from the ribosomal A to P and P to E sites, catalyzed by elongation factor EF-G. Overview The ribosomal P-site plays a vital role in all phases of translation. Initiation involves recognition of the start codon (AUG) by initiator tRNA in the P-site, elongation involves passage of many elongator tRNAs through the P site, termination involves hydrolysis of the mature polypeptide from tRNA bound to the P-site, and ribos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TRNA
Transfer ribonucleic acid (tRNA), formerly referred to as soluble ribonucleic acid (sRNA), is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes). In a cell, it provides the physical link between the genetic code in messenger RNA (mRNA) and the amino acid sequence of proteins, carrying the correct sequence of amino acids to be combined by the protein-synthesizing machinery, the ribosome. Each three-nucleotide codon in mRNA is complemented by a three-nucleotide anticodon in tRNA. As such, tRNAs are a necessary component of translation, the biological synthesis of new proteins in accordance with the genetic code. Overview The process of translation starts with the information stored in the nucleotide sequence of DNA. This is first transformed into mRNA, then tRNA specifies which three-nucleotide codon from the genetic code corresponds to which amino acid. Each mRNA codon is recognized by a particular type of tRNA, which docks to it along ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
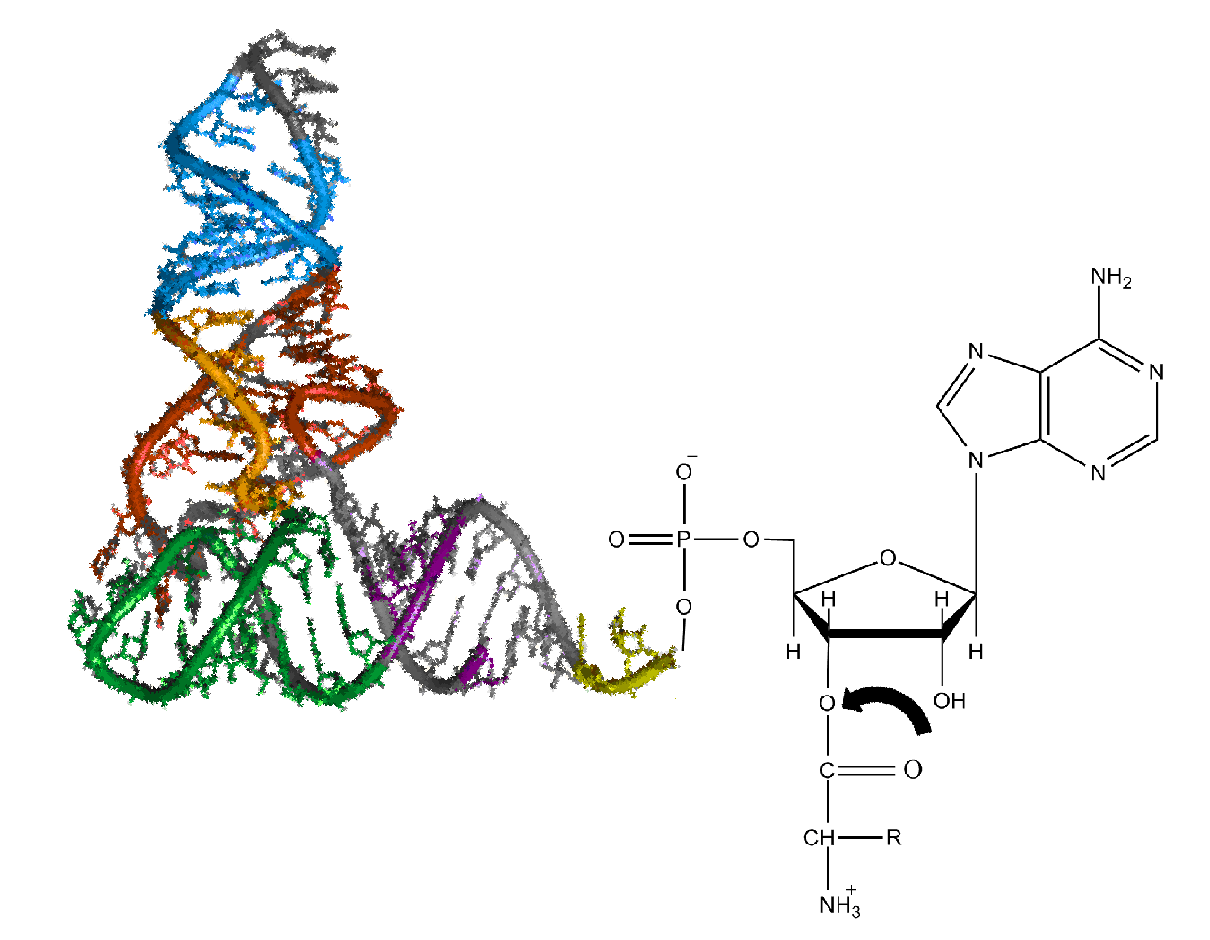
Ribosome
Ribosomes () are molecular machine, macromolecular machines, found within all cell (biology), cells, that perform Translation (biology), biological protein synthesis (messenger RNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA molecules to form polypeptide chains. Ribosomes consist of two major components: the small and large ribosomal subunits. Each subunit consists of one or more ribosomal RNA molecules and many ribosomal proteins (). The ribosomes and associated molecules are also known as the ''translational apparatus''. Overview The sequence of DNA that encodes the sequence of the amino acids in a protein is transcribed into a messenger RNA (mRNA) chain. Ribosomes bind to the messenger RNA molecules and use the RNA's sequence of nucleotides to determine the sequence of amino acids needed to generate a protein. Amino acids are selected and carried to the ribosome by transfer RNA (tRNA) molecules, which enter the riboso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Translation (biology)
In biology, translation is the process in living Cell (biology), cells in which proteins are produced using RNA molecules as templates. The generated protein is a sequence of amino acids. This sequence is determined by the sequence of nucleotides in the RNA. The nucleotides are considered three at a time. Each such triple results in the addition of one specific amino acid to the protein being generated. The matching from nucleotide triple to amino acid is called the genetic code. The translation is performed by a large complex of functional RNA and proteins called ribosomes. The entire process is called gene expression. In translation, messenger RNA (mRNA) is decoded in a ribosome, outside the nucleus, to produce a specific amino acid chain, or polypeptide. The polypeptide later protein folding, folds into an Activation energy, active protein and performs its functions in the cell. The polypeptide can also start folding during protein synthesis. The ribosome facilitates decoding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
A-site
The A-site (A for aminoacyl) of a ribosome is a binding site for charged t-RNA molecules during protein synthesis. One of three such binding sites, the A-site is the first location the t-RNA binds during the protein synthesis process, the other two sites being P-site (peptidyl) and E-site (exit). References Ribosomal RNA {{Genetics-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E-site
The E-site is the third and final binding site for t-RNA in the ribosome during translation, a part of protein synthesis. The "E" stands for exit, and is accompanied by the P-site (for peptidyl) which is the second binding site, and the A-site (aminoacyl), which is the first binding site. It is involved in cellular processes The cell is the basic structural and functional unit of all life, forms of life. Every cell consists of cytoplasm enclosed within a Cell membrane, membrane; many cells contain organelles, each with a specific function. The term comes from the .... References Further reading * * Ribosomal RNA {{Genetics-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Site
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may include other proteins (resulting in a protein–protein interaction), enzyme substrates, second messengers, hormones, or allosteric modulators. The binding event is often, but not always, accompanied by a conformational change that alters the protein's function. Binding to protein binding sites is most often reversible (transient and non-covalent), but can also be covalent reversible or irreversible. Function Binding of a ligand to a binding site on protein often triggers a change in conformation in the protein and results in altered cellular function. Hence binding site on protein are critical parts of signal transduction pathways. Types of ligands include neurotransmitters, toxins, neuropeptides, and steroid hormones. Binding site ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallography, crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information. X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences between various materials, especially minerals and alloys. The method has also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lincosamide
Lincosamides are a class of antibiotics, which include lincomycin, clindamycin, and pirlimycin. Structure Lincosamides consist of a pyrrolidine ring linked to a pyranose moiety (methylthio-lincosamide) via an amide bond. Hydrolysis of lincosamides, specifically lincomycin, splits the molecule into its building blocks of the sugar and proline moieties. Both of these derivatives can conversely be recombined into the drug itself or a derivative. Synthesis Biosynthesis of lincosamides occurs through a biphasic pathway, in which propylproline and methylthiolincosamide are independently synthesized immediately before condensation of the two precursor molecules. Condensation of the propylproline carboxyl group with the methylthiolincosamide amine group via an amide bond forms ''N''-demethyllincomycin. ''N''-Demethyllincomycin is subsequently methylated via ''S''-adenosyl methionine to produce lincomycin A. Lincomycin is naturally produced by bacteria species, namely '' Streptomyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrolide
Macrolides are a class of mostly natural products with a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. Macrolides belong to the polyketide class of natural products. Some macrolides have antibiotic or antifungal activity and are used as pharmaceutical drugs. Rapamycin is also a macrolide and was originally developed as an antifungal, but has since been used as an immunosuppressant drug and is being investigated as a potential longevity therapeutic. Macrolides are a diverse group with many members of very different properties: * Macrolides with 14-, 15-, or 16-membered rings and two attached sugar molecules are antibiotics that bind to bacterial ribosomes, the key representative being erythromycin. The term "macrolide antibiotics" tend to refer to just this class. * Some macrolides with very large (20+ membered) rings are immunosuppresants, the prototypical one being rapamycin. * Some 23-membered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleiotropy
Pleiotropy () is a condition in which a single gene or genetic variant influences multiple phenotypic traits. A gene that has such multiple effects is referred to as a ''pleiotropic gene''. Mutations in pleiotropic genes can impact several traits simultaneously, often because the gene product is used in various cell (biology), cells and affects different biological targets through shared signaling pathways. Pleiotropy can result from several distinct but potentially overlapping mechanisms, including gene pleiotropy, developmental biology, developmental pleiotropy, and selectional pleiotropy. Gene pleiotropy occurs when a gene product interacts with multiple proteins or catalyzes different reactions. Developmental pleiotropy refers to mutations that produce several phenotype, phenotypic effects during development. Selectional pleiotropy occurs when a single phenotype influences evolutionary fitness (biology), fitness in multiple ways (depending on factors such as age and sex). T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminoacyl-tRNA
Aminoacyl-tRNA (also aa-tRNA or charged tRNA) is tRNA to which its cognate amino acid is chemically bonded (charged). The aa-tRNA, along with particular elongation factors, deliver the amino acid to the ribosome for incorporation into the polypeptide chain that is being produced during translation. Alone, an amino acid is not the substrate necessary to allow for the formation of peptide bonds within a growing polypeptide chain. Instead, amino acids must be "charged" or aminoacylated with a tRNA to form their respective aa-tRNA. Every amino acid has its own specific aminoacyl-tRNA synthetase, which is utilized to chemically bind to the tRNA that it is specific to, or in other words, "cognate" to. The pairing of a tRNA with its cognate amino acid is crucial, as it ensures that only the particular amino acid matching the anticodon of the tRNA, and in turn matching the codon of the mRNA, is used during protein synthesis. In order to prevent translational errors, in which the wrong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cricket Paralysis Virus
Cricket paralysis virus (CrPV) is a paralytic disease affecting crickets. It was initially discovered in Australian field crickets ('' Teleogryllus commodus'' and '' Teleogryllus oceanicus'') by Carl Reinganum and his colleagues at the Victorian Plant Research Institute (Burnley, Melbourne, Australia). The disease spread rapidly through a breeding colony as well as through a laboratory population causing about 95% mortality. This was the first recorded isolate of the virus and is generally referred to as CrPVvic to distinguish it from subsequent isolates. Description The spheroidal, non-enveloped virus particles of CrPV are about 27 nm diameter in negatively-stained electron micrographs and contain a single piece of positive-sense ssRNA. The virion is composed of four capsid proteins with molecular masses generally reported to be 33, 31 and 30 kilodaltons with a minor VP4 protein of about 8 kDa. The particles resemble those of the mammalian picornaviruses but CrPV virions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |