|
Oxycation
In chemistry, an oxycation is an ion with the generic formula (where A represents a chemical element and O represents an oxygen atom). They usually end with the suffix "-ium" or "-yl". However, the International Union of Pure and Applied Chemistry standard book on nomenclature of inorganic chemistry does not mention "oxycation". Examples * * and See also *Oxyanion An oxyanion, or oxoanion, is an ion with the generic formula (where A represents a chemical element and O represents an oxygen atom). Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determine ... * List of aqueous ions by element References Cations {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Aqueous Ions By Element
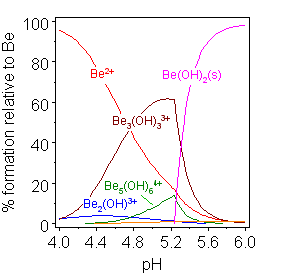
This table lists the ionic species that are most likely to be present, depending on pH, in aqueous solutions of binary salts of metal ions. The existence must be inferred on the basis of indirect evidence provided by modelling with experimental data or by analogy with structures obtained by X-ray crystallography. Introduction When a salt of a metal ion, with the generic formula MXn, is dissolved in water, it will dissociate into a cation and anions. : MX_n \rarr M^(aq) +nX^-(aq) (aq) signifies that the ion is aquated, with cations having a chemical formula (H2O)psup>q+ and anions whose state of aquation is generally unknown. For convenience (aq) is not shown in the rest of this article as the ''number'' of water molecules that are attached to the ions is irrelevant in regard to hydrolysis. This reaction occurs quantitatively with salts of the alkali-metals at low to moderate concentrations. With salts of divalent metal ions, the aqua-ion will be subject to a dissociation reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its atomic nucleus, nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements form Homonuclear molecule, molecules of atoms of said element only: e.g. atoms of hydrogen (H) form Diatomic molecule, diatomic molecules (H). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxyanion
An oxyanion, or oxoanion, is an ion with the generic formula (where A represents a chemical element and O represents an oxygen atom). Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound . The structures of condensed oxyanions can be rationalized in terms of AO''n'' polyhedral units with sharing of corners or edges between polyhedra. The oxyanions (specifically, phosphate and polyphosphate esters) adenosine monophosphate ( AMP), adenosine diphosphate ( ADP) and adenosine triphosphate (ATP) are important in biology. Monomeric oxyanions The formula of monomeric oxyanions, , is dictated by the oxidation state of the element A and its position in the periodic table. Elements of the first row are limited to a maximum coordination number of 4. However, none of the first row elements has a monomeric oxyanion with that coordination number. Instead, c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cations
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− (hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |