|
Oxohalides
In chemistry, oxohalides or oxyhalides are a group of chemical compounds with the chemical formula , where X is a halogen, and A is an element different than O and X. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I). The element A may be a main group element, a transition element, a rare earth element or an actinide. Molecular oxohalides are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. The term ''oxohalide'', or ''oxyhalide'', also refers to ionic oxohalides with the same overall chemical formula, but having an Ionic crystal, ionic structure. There are minerals that are ionic oxohalides. Synthesis Oxohalides can be seen as compounds intermediate between oxides and halides. There are three general methods of synthesis: *Partial oxidation of a halide: *: **In this example, the oxidation state increases by two and the electrical charge is unchanged. *Partial ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromyl Chloride
Chromyl chloride is an inorganic compound with the formula CrO2Cl2. It is a reddish brown compound that is a volatile liquid at room temperature, which is unusual for transition metal compounds. It is the dichloride of chromic acid. Preparation Chromyl chloride can be prepared by the reaction of potassium chromate or potassium dichromate with hydrogen chloride in the presence of concentrated sulfuric acid, followed by distillation. :K2Cr2O7 + 6 HCl → 2 CrO2Cl2 + 2 KCl + 3 H2O The sulfuric acid serves as a dehydration agent. It can also be prepared directly by exposing chromium trioxide to anhydrous hydrogen chloride gas. :CrO3 + 2 HCl ⇌ CrO2Cl2 + H2O Usage Test for the presence of chlorides The chromyl chloride test involves heating a sample suspected to contain chlorides with potassium dichromate and concentrated sulfuric acid. If a chloride is present then chromyl chloride forms which is indicated by the evolution of red smoke. No similar compound is formed in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CrO2Cl2
Chromyl chloride is an inorganic compound with the formula CrO2Cl2. It is a reddish brown compound that is a volatile liquid at room temperature, which is unusual for transition metal compounds. It is the dichloride of chromic acid. Preparation Chromyl chloride can be prepared by the reaction of potassium chromate or potassium dichromate with hydrogen chloride in the presence of concentrated sulfuric acid, followed by distillation. :K2Cr2O7 + 6 HCl → 2 CrO2Cl2 + 2 KCl + 3 H2O The sulfuric acid serves as a dehydration agent. It can also be prepared directly by exposing chromium trioxide to anhydrous hydrogen chloride gas. :CrO3 + 2 HCl ⇌ CrO2Cl2 + H2O Usage Test for the presence of chlorides The chromyl chloride test involves heating a sample suspected to contain chlorides with potassium dichromate and concentrated sulfuric acid. If a chloride is present then chromyl chloride forms which is indicated by the evolution of red smoke. No similar compound is formed in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydron
In chemistry, the hydron, informally called proton, is the cationic form of atomic hydrogen, represented with the symbol . The general term "hydron", endorsed by IUPAC, encompasses cations of hydrogen regardless of isotope: thus it refers collectively to protons (H) for the protium isotope, deuterons (H or D) for the deuterium isotope, and tritons (H or T) for the tritium isotope. Unlike most other ions, the hydron consists only of a bare atomic nucleus. The negatively charged counterpart of the hydron is the hydride anion, . Properties Solute properties Other things being equal, compounds that readily donate hydrons (Brønsted acids, see below) are generally polar, hydrophilic solutes and are often soluble in solvents with high relative static permittivity (dielectric constants). Examples include organic acids like acetic acid (CHCOOH) or methanesulfonic acid (CHSOH). However, large nonpolar portions of the molecule may attenuate these properties. Thus, as a result of its alk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pronunciation of the word "chloride" is . Chloride salts such as sodium chloride are often soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Other examples of ionic chlorides include potassium chloride (), calcium chloride (), and ammonium chloride (). Examples of covalent chlorides include methyl chloride (), carbon tetrachloride (), sulfuryl chloride (), and monochloramine (). Electronic properties A chloride ion (diameter 167 pm) is much larger than a chlorine atom (diameter 99 pm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
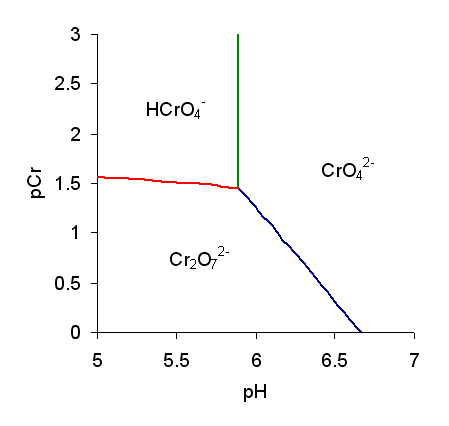
Chromate And Dichromate
Chromate salts contain the chromate anion, . Dichromate salts contain the dichromate anion, . They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible. Chemical properties Potassium-chromate-sample.jpg, Potassium chromate Potassium-dichromate-sample.jpg, Potassium dichromate Chromates react with hydrogen peroxide, giving products in which peroxide, , replaces one or more oxygen atoms. In acid solution the unstable blue peroxo complex Chromium(VI) oxide peroxide, , is formed; it is an uncharged covalent molecule, which may be extracted into ether. Addition of pyridine results in the formation of the more stable complex . Acid–base properties In aqueous solution, chromate and dichromate anions exist in a chemical equilibrium. : The predominance diagram shows that the position of the equilibrium depends on both pH and the analytical concentration o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VOCl3
Vanadium oxytrichloride is the inorganic compound with the formula VOCl3. This yellow distillable liquid hydrolyzes readily in air. It is an oxidizing agent. It is used as a reagent in organic synthesis. Samples often appear red or orange owing to an impurity of vanadium tetrachloride. Properties VOCl3 is a vanadium compound with vanadium in the +5 oxidation state and as such is diamagnetic. It is tetrahedral with O-V-Cl bond angles of 111° and Cl-V-Cl bond angles of 108°. The V-O and V-Cl bond lengths are 157 and 214 pm, respectively. VOCl3 is highly reactive toward water and evolves HCl upon standing. It is soluble in nonpolar solvents such as benzene, CH2Cl2, and hexane. In some aspects, the chemical properties of VOCl3 and POCl3 are similar. One distinction is that VOCl3 is a strong oxidizing agent, whereas the phosphorus compound is not. Neat VOCl3 is the usual chemical shift standard for 51V NMR spectroscopy. Preparation VOCl3 arises by the chlorination of V2O5 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
V2O5
Vanadium(V) oxide (''vanadia'') is the inorganic compound with the formula V2 O5. Commonly known as vanadium pentoxide, it is a dark yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst. The mineral form of this compound, shcherbinaite, is extremely rare, almost always found among fumaroles. A mineral trihydrate, V2O5·3H2O, is also known under the name of navajoite. Chemical properties Reduction to lower oxides Upon heating a mixture of vanadium(V) oxide and vanadium(III) oxide, comproportionation occurs to give vanadium(IV) oxide, as a deep-blue solid: :V2O5 + V2O3 → 4 VO2 The reduction can also be effected by oxalic acid, carbon monoxide, and sulfur dioxide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure ionic bonding, many covalent bonds exhibit a strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on Electronegativities of the elements (data page), the choice of electronegativity scale used in their calculation. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
POCl3
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula . It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters. Structure Like phosphate, is tetrahedral in shape. It features three P−Cl bonds and one strong P–O bond, with an estimated bond dissociation energy of 533.5 kJ/mol. Unlike in the case of , the Schomaker-Stevenson rule predicts appropriate bond length for the P–O bond only if the P–O bond is treated as a double bond, P=O. More modern treatments explain the tight P–O bond as a combination of lone pair transfer from the phosphorus to the oxygen atom and a dative ''π'' back-bond that produces an effective + −configuration. Phosphoryl chloride exists as neutral molecules in the solid, liquid and gas states. This is un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |