|
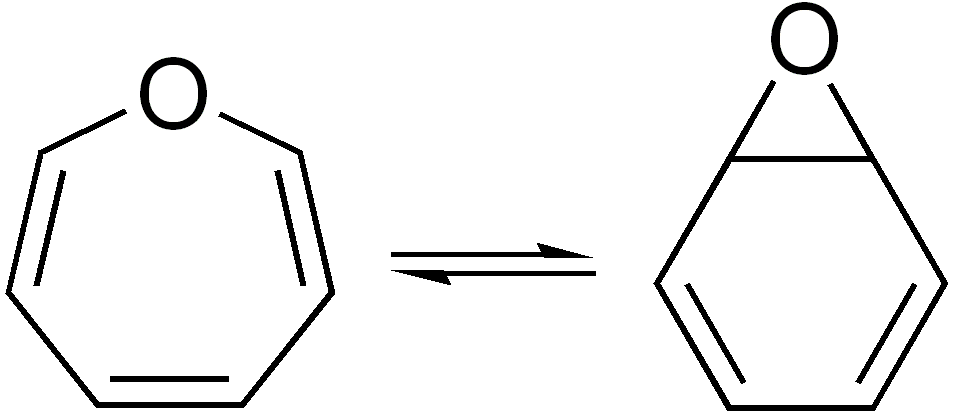
Oxepin
Oxepine is an oxygen-containing heterocycle consisting of a seven-membered ring with three double bonds. The parent C6H6O exists as an equilibrium mixture with benzene oxide. The oxepin–benzene oxide equilibrium is affected by the ring substituents. A related dimethyl derivative exists mainly as the oxepine isomer, an orange liquid. Oxepine is an intermediate in the oxidation of benzene by the cytochrome P450 (CYP). Other arene oxide In chemistry, an arene oxide is an epoxide of an arene. Two important families of arene oxides are benzene oxides and naphthalene oxides as these are intermediates in the oxidative degradation of benzene and naphthalene, two common pollutants. Be ...s are metabolites of the parent arene. : References {{Commons category, Oxepin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arene Oxide
In chemistry, an arene oxide is an epoxide of an arene. Two important families of arene oxides are benzene oxides and naphthalene oxides as these are intermediates in the oxidative degradation of benzene and naphthalene, two common pollutants. Benzopyrene is also converted to an epoxide, (+)-benzo[a]pyrene-7,8-epoxide. Selected reactions Benzene oxide (C6H6O) exists as an chemical equilibrium, equilibrium mixture with the seven-membered ring (chemistry), ring oxepin, which has three double bonds. They are valence isomers and in equilibrium via disrotatory 6π ring closing and opening. Arene oxides are highly reactive. Benzene oxide and naphthalene-1,2-oxide hydrate to give dihydroxydihydrobenzene and 1,2-dihydroxydihydronaphthalene, respectively. The hydration is catalyzed by epoxide hydrolase enzymes. Dehydration of these diols, which is driven by rearomatization, gives phenol and 1-naphthol. Oxidation of 1,2-dihydroxydihydronaphthalene, catalyzed by dihydrodiol dehydrogenas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of organic heterocyclic chemistry focuses especially on organic unsaturated derivatives, and the preponderance of work and applications involves unstrained organic 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of organic heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexene Oxide
Cyclohexene oxide is a cycloaliphatic epoxide. It can react in cationic polymerization to poly(cyclohexene oxide). As cyclohexene is monovalent, poly(cyclohexene oxide) is a thermoplastic. Production Cyclohexene oxide is produced in epoxidation reaction from cyclohexene. The epoxidation can take place either in a homogeneous reaction by peracids or heterogeneous catalysis (e.g. silver and molecular oxygen). : 250px In industrial production the heterogeneously catalyzed synthesis is preferred because of better atom economy, a simpler separation of the product and easier recycling of catalyst. A short overview and an investigation of the oxidation of cyclohexene by hydrogen peroxide is given in the literature. In recent times the catalytic oxidation of cyclohexene by (immobilized) metalloporphyrin complexes has been found to be an efficient way. In laboratory, cyclohexene oxide can also be prepared by reacting cyclohexene with magnesium monoperoxyphthalate (MMPP) in a mixture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring (chemistry)
In chemistry, a ring is an ambiguous term referring either to a simple cycle of atoms and bonds in a molecule or to a connected set of atoms and bonds in which every atom and bond is a member of a cycle (also called a ring system). A ring system that is a simple cycle is called a monocycle or simple ring, and one that is not a simple cycle is called a polycycle or polycyclic ring system. A simple ring contains the same number of sigma bonds as atoms, and a polycyclic ring system contains more sigma bonds than atoms. A molecule containing one or more rings is called a cyclic compound, and a molecule containing two or more rings (either in the same or different ring systems) is termed a polycyclic compound. A molecule containing no rings is called an acyclic or open-chain compound. Homocyclic and heterocyclic rings A homocycle or homocyclic ring is a ring in which all atoms are of the same chemical element. A heterocycle or heterocyclic ring is a ring containing atoms of at least ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were introduced in chemical notation by Russian chemist Alexander Butlerov. Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two. Double bonds are also electron-rich, which makes them potentially more reactive in the presence of a strong electron acceptor (as in addition reactions of the halogens). File:Ethene structural.svg, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the Thermodynamic system, system. This state results when the forward reaction proceeds at the same rate as the Reversible reaction, reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. It is the subject of study of ''equilibrium chemistry''. Historical introduction The Concept learning, concept of chemical equilibrium was developed in 1803, after Claude Louis Berthollet, Berthollet found that some chemical reactions are Reversible reaction, reversible. For any reaction mixture to exist at equilibrium, the reaction rate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. The suffix ''-yl'' is used when naming organic compounds that contain a single bond replacing one hydrogen; ''-ylidene'' and ''-ylidyne'' are used with double bonds and triple bonds, respectively. In addition, when naming hydrocarbons that contain a substituent, positional numbers are used to indicate which carbon atom the substituent attaches to when such information is needed to distinguish between isomers. Substituents can be a combination of the inductive effect and the mesomeric effect. Such effects are also described as electron-rich and electron withdrawing. Additional steric effects result from the volume occupied by a substituent. The phrases ''most-substituted'' and ''least-substituted'' are frequently used to describe or compare molecules that are products of a chemical reaction. In this terminology, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (P450s or CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. In mammals, these enzymes oxidize steroids, fatty acids, xenobiotics, and participate in many biosyntheses. By hydroxylation, CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted. P450s are, in general, the terminal oxidase enzymes in electron transfer chains, broadly categorized as P450-containing systems. The term "P450" is derived from the spectrophotometry, spectrophotometric peak at the wavelength of the absorption spectroscopy, absorption maximum of the enzyme (450 nanometre, nm) when it is in the redox, reduced state and complexed with carbon monoxide. Most P450s require a protein partner to deliver one or more electrons to reduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |