|
Nitrosylsulfuric Acid
Nitrosylsulfuric acid is the chemical compound with the formula . It is a colourless solid that is used industrially in the production of caprolactam, and was formerly part of the lead chamber process for producing sulfuric acid. The compound is the mixed anhydride of sulfuric acid and nitrous acid. In organic chemistry, it is used as a reagent for nitrosating, as a diazotizing agent, and as an oxidizing agent. Synthesis and reactions A typical procedure entails dissolving sodium nitrite in cold sulfuric acid: : It can also be prepared by the reaction of nitric acid and sulfur dioxide. This procedure generates the nitrosylsulfuric acid as an intermediate en route to NOCl. is used in organic chemistry to prepare diazonium salts from amines, for example in the Sandmeyer reaction. Related NO-delivery reagents include nitrosonium tetrafluoroborate and nitrosyl chloride. In industry, the nitrosodecarboxylation reaction between nitrosylsulfuric acid and cyclohexanecarboxylic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula . It is a colorless, odorless, and Viscosity, viscous liquid that is Miscibility, miscible with water. Pure sulfuric acid does not occur naturally due to its Dehydration reaction, strong affinity to water vapor; it is Hygroscopy, hygroscopic and readily absorbs water vapor from the Atmosphere of Earth, air. Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is releas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazonium Salt
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent, compound where R is hydrogen, is diazenylium. Structure and general properties Arene derivatives According to X-ray crystallography the linkage is linear in typical diazonium salts. The bond distance in benzenediazonium tetrafluoroborate is 1.083(3) Å, which is almost identical to that for dinitrogen molecule (N≡N). The linear free energy constants σm and σp indicate that the diazonium group is strongly electron-withdrawing. Thus, the diazonio-substituted phenols and benzoic acids have greatly reduced p''K''a values compared to their unsubstituted counterparts. The p''K''a of phenolic proton of 4-hydroxybenzenediazonium is 3.4, versus 9.9 for phenol itself. In other words, the diazonium group raises the ionization constant ''K'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Esters
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry of the isolated anion is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosyl Compounds
In organic chemistry, nitroso refers to a functional group in which the nitric oxide () group is attached to an organic moiety. As such, various nitroso groups can be categorized as ''C''-nitroso compounds (e.g., nitrosoalkanes; ), ''S''-nitroso compounds ( nitrosothiols; ), ''N''-nitroso compounds (e.g., nitrosamines, ), and ''O''-nitroso compounds (alkyl nitrites; ). Synthesis Nitroso compounds can be prepared by the reduction of nitro compounds or by the oxidation of hydroxylamines. Ortho-nitrosophenols may be produced by the Baudisch reaction. In the Fischer–Hepp rearrangement, aromatic 4-nitrosoanilines are prepared from the corresponding nitrosamines. Properties Nitrosoarenes typically participate in a monomer–dimer equilibrium. The azobenzene ''N'',''N'-''dioxide (Ar(–O)N+=+N(O–)Ar) dimers, which are often pale yellow, are generally favored in the solid state, whereas the deep-green monomers are favored in dilute solution or at higher temperatures. They exi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxoacids
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce the H+ cation and the anion of the acid. Description Under Lavoisier's original theory, all acids contained oxygen, which was named from . It was later discovered that some acids, notably hydrochloric acid, did not contain oxygen and so acids were divided into oxo-acids and these new hydroacids. All oxyacids have the acidic hydrogen bound to an oxygen atom, so bond strength (length) is not a factor, as it is with binary nonmetal hydrides. Rather, the electronegativity of the central atom and the number of oxygen atoms determine oxyacid acidity. For oxyacids with the same central atom, acid strength increases with the number of oxygen atoms attached to it. With the same number of oxygen atoms attached to it, acid strength increases with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acids
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Arrhenius acids. Brønsted and Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and react with bases and certain metals (like calcium) to form salts. The word ''acid'' is derived from the Latin , meaning 'sour'. An aqueous solution of an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Anhydrides
An organic acid anhydride is an acid anhydride that is also an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word ''acid'' in the name of the parent carboxylic acid by the word ''anhydride''. Thus, (CH3CO)2O is called ''acetic anhydride.'' ''Mixed'' (or ''unsymmetrical'') acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride"). One or both acyl groups of an acid anhydride ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SNIA S
SNIA or Snia may refer to: * Storage Networking Industry Association, a trade organization focusing on computer storage * SNIA S.p.A. Snia, stylized as SNIA, (later SNIA Viscosa and finally SNIA BDG Srl) was an Italian language, Italian firm located in Milan that manufactured defence products, textiles, chemicals, perfumes, and corrugated paper among other products. History Th ..., a former Italian manufacturing firm * Snia Milano, an athletics club {{Disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexanecarboxylic Acid
Cyclohexanecarboxylic acid is the organic compound with the formula C6H11CO2H. It is the carboxylic acid of cyclohexane. It is a colorless oil that crystallizes near room temperature.. Preparation and reactions It is prepared by hydrogenation of benzoic acid. Cyclohexanecarboxylic acid is a precursor to the nylon-6 precursor caprolactam via its reaction with nitrosylsulfuric acid. It can also be oxidized to cyclohexene. Cyclohexanecarboxylic acid exhibits the reactions typical of carboxylic acids, including its conversion to the acid chloride cyclohexanecarbonyl chloride. Related compounds Derivatives related to cyclohexanecarboxylic acid include: * abscisic acid * buciclic acid * chlorogenic acid * chorismic acid * dicyclomine * quinic acid * shikimic acid * tranexamic acid Tranexamic acid is a medication used to treat or prevent excessive blood loss from major trauma, postpartum bleeding, surgery, tooth removal, nosebleeds, and heavy menstruation. It is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
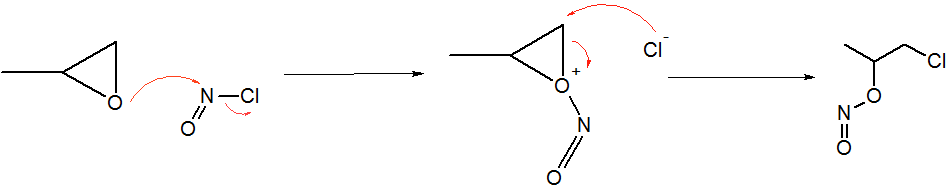
Nitrosyl Chloride
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound. Structure and synthesis The molecule is bent. A double bond exists between N and O (distance = 1.16 Å) and a single bond between N and Cl (distance = 1.96 Å). The O=N–Cl angle is 113°. Production Nitrosyl chloride can be produced in many ways. * Combining nitrosylsulfuric acid and HCl affords the compound. This method is used industrially. :HCl + NOHSO4 → H2SO4 + NOCl * A more convenient laboratory method involves the (reversible) dehydration of nitrous acid by HCl : HNO2 + HCl → H2O + NOCl * By the direct combination of chlorine and nitric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosonium Tetrafluoroborate
Nitrosonium tetrafluoroborate, also called nitrosyl tetrafluoroborate, is a chemical compound with the chemical formula NOBF4. This colourless solid is used in organic synthesis as a nitrosating agent, diazotizing agent and a mild oxidant. NOBF4 is the nitrosonium salt of fluoroboric acid, and is composed of a nitrosonium cation, Osup>+, and a tetrafluoroborate anion, F4sup>−. Reactions The dominant property of NOBF4 is the oxidizing power and electrophilic character of the nitrosonium cation. It forms colored charge transfer complexes with hexamethylbenzene and with 18-crown-6. The latter, a deep yellow species, provides a means to dissolve NOBF4 in dichloromethane. Nitrosonium tetrafluoroborate may be used to prepare metal salts of the type II(CH3CN)''x''F4sub>2 (M = Cr, Mn, Fe, Co, Ni, Cu). The nitrosonium cation acts as the oxidizer, itself being reduced to nitric oxide gas: : With ferrocene the ferrocenium tetrafluoroborate is formed. In its infrared spectrum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |