|
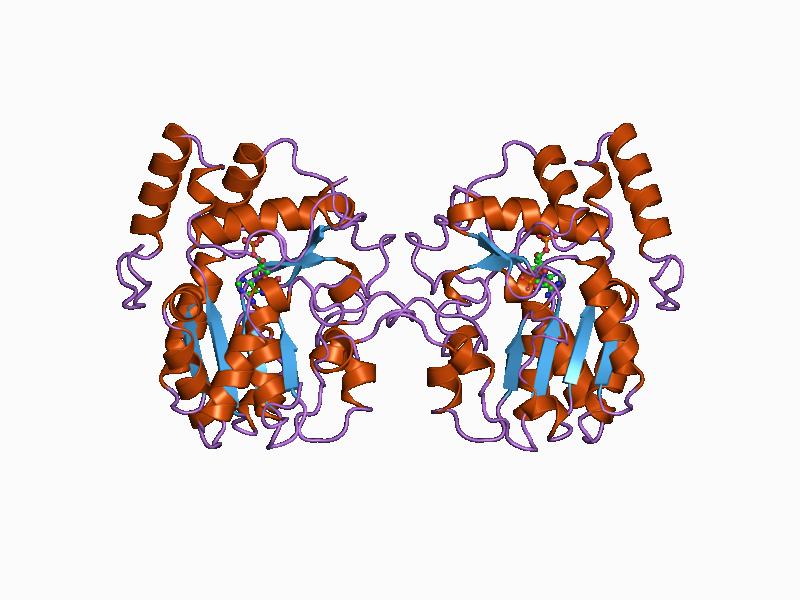
N-acetylglucosamine-1-phosphate Transferase
N-acetylglucosamine-1-phosphate transferase (GlcNAc-1-phosphotransferase) is a transferase enzyme. Function It is made up of two alpha (α), two betas (β), and two gammas (γ) subunits. ''GNPTAB'' produces the alpha and beta subunits, '' GNPTG'' produces the gamma subunit. GlcNAc-1-phosphotransferase functions to prepare newly made enzymes for lysosome transportation (lysosomal hydrolases to the lysosome). Lysosomes, a part of an animal cell, helps break down large molecules into smaller ones that can be reused. GlcNAc-1-phosphotransferase phosphorylates carbon 6 of one or more mannosyl residues of ''N''-linked glycoproteins being processed in the Golgi apparatus. UDP-GLcNAc provides the phosphate in a reaction catalysed by this enzyme. M6P acts as an indicator of whether a hydrolase should be transported to the lysosome or not. Once a hydrolase indicates an M6P, it can be transported to a lysosome. Surprisingly some lysosomal enzymes are only tagged at a rate of 5% or lower. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transferase
In biochemistry, a transferase is any one of a class of enzymes that catalyse the transfer of specific functional groups (e.g. a methyl or glycosyl group) from one molecule (called the donor) to another (called the acceptor). They are involved in hundreds of different biochemical pathways throughout biology, and are integral to some of life's most important processes. Transferases are involved in myriad reactions in the cell. Three examples of these reactions are the activity of coenzyme A (CoA) transferase, which transfers thiol esters, the action of N-acetyltransferase, which is part of the pathway that metabolizes tryptophan, and the regulation of pyruvate dehydrogenase (PDH), which converts pyruvate to acetyl CoA. Transferases are also utilized during translation. In this case, an amino acid chain is the functional group transferred by a peptidyl transferase. The transfer involves the removal of the growing amino acid chain from the tRNA molecule in the A-site of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GNPTG
GNPTG (“N-acetylglucosamine-1-phosphate transferase, gamma subunit.”) is a gene in the human body. It is one of three genes that were found to correlate with stuttering. Function The GNPTG gene codes instructions for making the gamma subunit of an enzyme called GlcNAc-1-phosphotransferase (also called N-acetylglucosamine-1-phosphate transferase). This enzyme is made up of two alpha (α), two beta (β), and two gamma (γ) subunits. GNPTAB produces the alpha and beta subunits. GlcNAc-1-phosphotransferase functions to prepare newly made enzymes for lysosome transportation (lysosomal hydrolases to the lysosome). Lysosome A lysosome () is a membrane-bound organelle that is found in all mammalian cells, with the exception of red blood cells (erythrocytes). There are normally hundreds of lysosomes in the cytosol, where they function as the cell’s degradation cent ...s, a part of an animal cells, helps break down large molecules into smaller ones that can be reused. GlcNAc-1- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysosome
A lysosome () is a membrane-bound organelle that is found in all mammalian cells, with the exception of red blood cells (erythrocytes). There are normally hundreds of lysosomes in the cytosol, where they function as the cell’s degradation center. Their primary responsibility is catabolic degradation of proteins, polysaccharides and lipids into their respective building-block molecules: amino acids, monosaccharides, and free fatty acids. The breakdown is done by various enzymes, for example proteases, glycosidases and lipases. With an acidic lumen limited by a single-bilayer lipid membrane, the lysosome holds an environment isolated from the rest of the cell. The lower pH creates optimal conditions for the over 60 different Hydrolase, hydrolases inside. Lysosomes receive extracellular particles through endocytosis, and intracellular components through autophagy. They can also fuse with the plasma membrane and secrete their contents, a process called lysosomal exocytosis. After ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
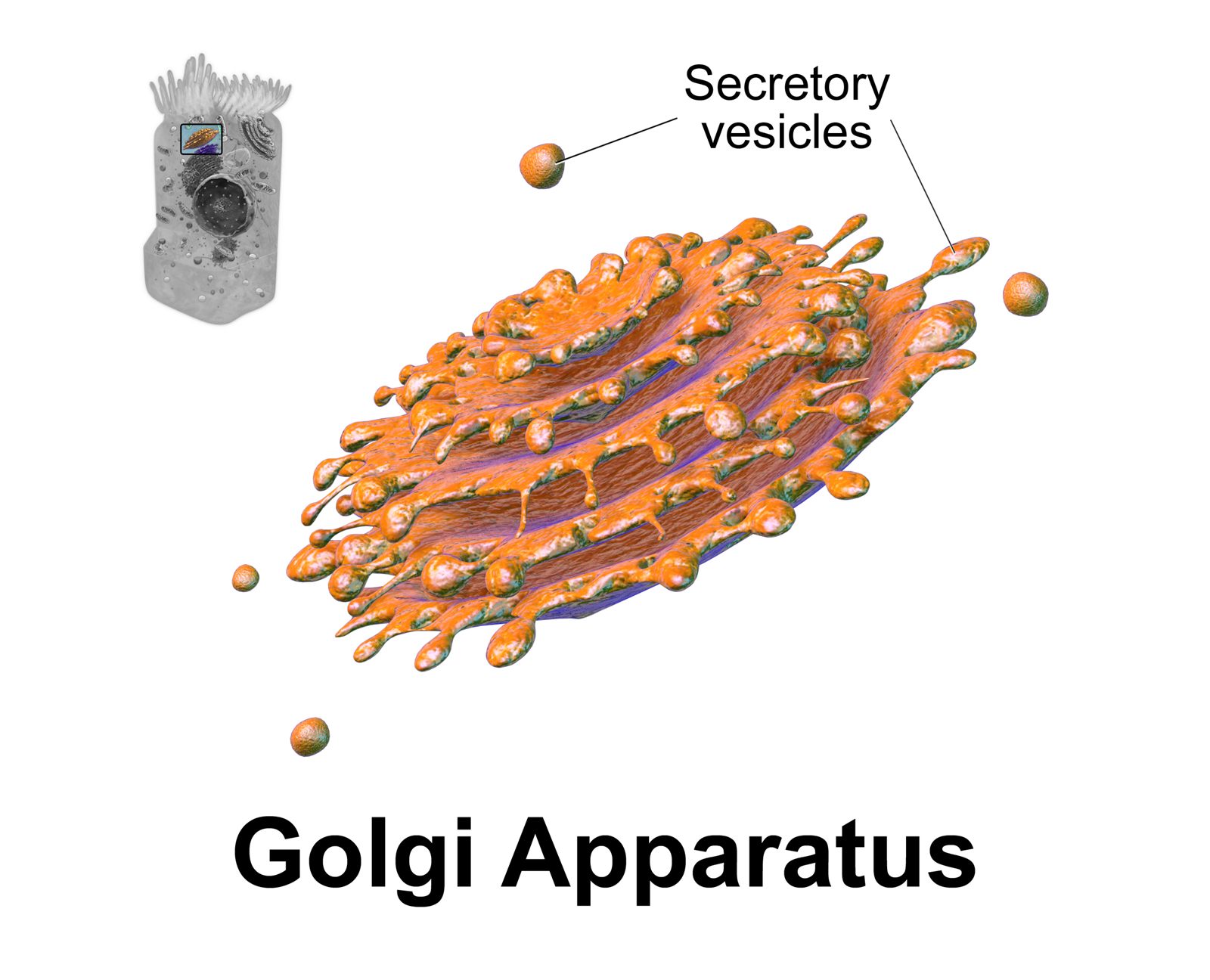
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic Cell (biology), cells. Part of the endomembrane system in the cytoplasm, it protein targeting, packages proteins into membrane-bound Vesicle (biology and chemistry), vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and Endocytosis, endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. The Golgi apparatus was identified in 1898 by the Italian biologist and pathologist Camillo Golgi. The organelle was later named after him in the 1910s. Discovery Because of its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
I-cell Disease
Inclusion-cell (I-cell) disease, also referred to as mucolipidosis II (ML II), is part of the lysosomal storage disease family and results from a defective phosphotransferase (an enzyme of the Golgi apparatus). This enzyme transfers phosphate to mannose residues on specific proteins. Mannose-6-phosphate serves as a marker for proteins to be targeted to lysosomes within the cell. Without this marker, proteins are instead secreted outside the cell, which is the default pathway for proteins moving through the Golgi apparatus. Lysosomes cannot function without these proteins, which function as catabolic enzymes for the normal breakdown of substances (e.g. oligosaccharides, lipids, and glycosaminoglycans) in various tissues throughout the body (i.e. fibroblasts). As a result, a buildup of these substances occurs within lysosomes because they cannot be degraded, resulting in the characteristic I-cells, or "inclusion cells" seen microscopically. In addition, the defective lysosomal enz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudo-Hurler Polydystrophy
Pseudo-Hurler polydystrophy, also referred to as mucolipidosis III (ML III), is a lysosomal storage disease closely related to I-cell disease (ML II). This disorder is called Pseudo-Hurler because it resembles a mild form of Hurler syndrome, one of the mucopolysaccharide (MPS) diseases. Signs and symptoms Symptoms of ML III are often not noticed until the child is 3–5 years of age. Patients with ML III are generally of normal intelligence (trait) or have only mild mental retardation (intelligence is challenged) instead of using the mental retardation classification. These patients usually have skeletal abnormalities, coarse facial features, short height, corneal clouding, carpal tunnel syndrome, aortic valve disease and mild enlargement of organs. Some children with severe forms of this disease do not live beyond childhood. However, there is a great variability among patients – there are diagnosed individuals with ML III living in their sixties. Pathophysiology As in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |