|
N,N-Dimethylethanolamine Bitartrate
''N'',''N''-Dimethylethanolamine bitartrate or deanol bitartrate is an organic compound with the chemical formula . It is a white powder. Modern texts refer to the ''N'',''N''-dimethylethanolamine salt of the natural form of tartaric acid, that is, the salt called ''N'',''N''-dimethylethanolamine dextrobitartrate, ''N'',''N''-dimethylethanolamine (2''R'',3''R'')-bitartrate or ''N'',''N''-dimethylethanolamine L-(+)-bitartrate. Chemistry ''N'',''N''-Dimethylethanolamine bitartrate is a ''N'',''N''-dimethylethanolamine salt of tartaric acid. ''N'',''N''-Dimethylethanolamine bitartrate contains tertiary ammonium cations (dimethyl(2-hydroxyethyl)ammonium ) and bitartrate anions (). Tertiary ammonium cation is a cation in which three hydrogen atoms of ammonium are replaced with organyl groups. In this compound, the three substituents of ammonium are two methyl groups () and one 2-hydroxy ethyl group (). The bitartrate anion is chiral (there are left, right and meso forms of bitartrate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, Volatility (chemistry), volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a Precursor (chemistry), precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl ''tert''-butyl ether, methyl benzoate, anisole, peroxyacids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylethanolamine
Dimethylethanolamine (DMAE or DMEA) is an organic compound with the formula . It is bifunctional, containing both a tertiary amine and primary alcohol functional groups. It is a colorless viscous liquid. It is used in skin care products for improving skin tone and also taken orally as a nootropic. It is prepared by the ethoxylation of dimethylamine. Industrial uses Dimethylaminoethanol is used as a curing agent for polyurethanes and epoxy resins. It is a precursor to other chemicals, such as the nitrogen mustard 2-dimethylaminoethyl chloride. The acrylate ester, dimethylaminoethyl acrylate is used as a flocculating agent. Related compounds are used in gas purification, e.g. removal of hydrogen sulfide from sour gas streams. Human uses The bitartrate salt of DMAE, i.e. ''N'',''N''-dimethylethanolamine bitartrate, is sold as a dietary supplement. It is a white powder providing 37% DMAE. Animal tests show possible benefit for improving spatial memory and working memory. See ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Group
In organic chemistry, an ethyl group (abbr. Et) is an alkyl substituent with the formula , derived from ethane (). ''Ethyl'' is used in the International Union of Pure and Applied Chemistry The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...'s nomenclature of organic chemistry for a saturated two-carbon moiety in a molecule, while the prefix "''eth-''" is used to indicate the presence of two carbon atoms in the molecule. Ethylation Ethylation is the formation of a compound by introduction of the ethyl group. The most widely practiced example of this reaction is the ethylation of benzene with ethylene to yield ethylbenzene, a precursor to styrene, which is a precursor to polystyrene. Approximately 24.7 million tons of ethylbenzene were produced in 1999. :: Many ethyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For exam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
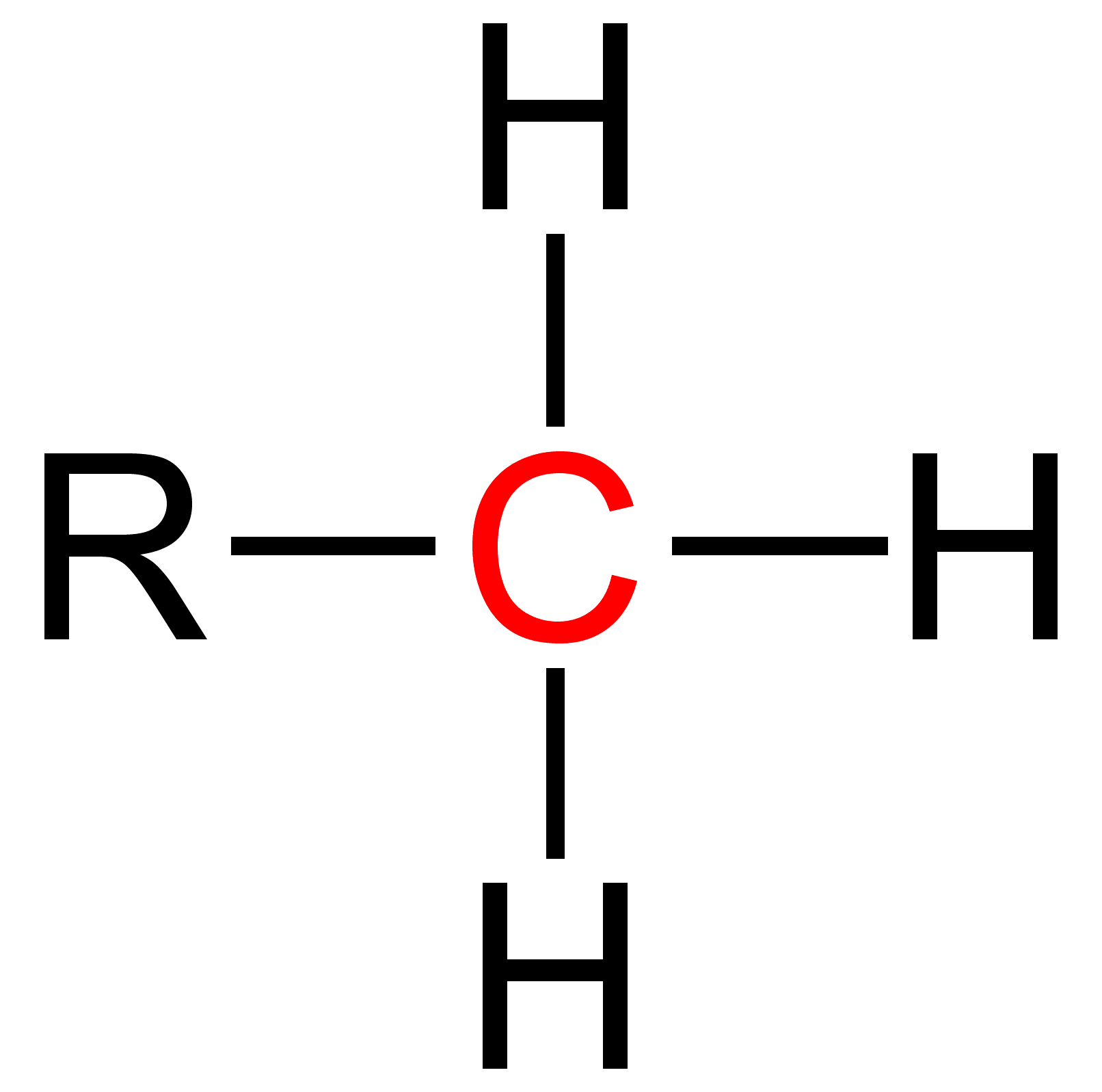
Organyl
In organic and organometallic chemistry, an organyl group (commonly denoted by the letter " R") is an organic substituent with one (sometimes more) free valence electron(s) at a carbon atom.. The term is often used in chemical patent literature to protect claims over a broad scope. Examples * Acetonyl group * Acyl group (e.g. acetyl group, benzoyl group) * Alkyl group (e.g., methyl group, ethyl group) * Alkenyl group (e.g., vinyl group, allyl group) * Alkynyl group ( propargyl group) * Benzyloxycarbonyl group (Cbz) * '' tert'' -butoxycarbonyl group (Boc) * Carboxyl group In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g. ... References Functional groups {{organic-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleus) to ammonia (). Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by Organic compound, organic or other groups (indicated by R). Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, human impact in recent years could have an effect on the biological communities that depend on it. Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted–Lowry acid–base theory, Brønsted acids (proton donors): : The ammonium ion is mildly acidic, reacting with Brønsted bases to return ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− (hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bitartrate
, Section2 = {{Chembox Properties , C=4 , H=5 , O=6 , Formula_Charge = − , ConjugateAcid = Tartaric acid , ConjugateBase = Tartrate Bitartrate is an anion which is the conjugate base of tartaric acid. It may also refer to any salt In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ... or monoester of tartaric acid. Some examples of bitartrate salts include: * Choline bitartrate * Cysteamine bitartrate * Dihydrocodeine bitartrate * Dimethylaminoethanol bitartrate * Hydrocodone bitartrate * Metaraminol bitartrate * Norepinephrine bitartrate * Potassium bitartrate * Sodium bitartrate References Tartrates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− ( hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary (chemistry)
Tertiary is a term used in organic chemistry to classify various types of compounds (e. g. alcohols, alkyl halides, amines) or reactive intermediates (e. g. alkyl radicals, carbocations). See also * Primary (chemistry) Primary is a term used in organic chemistry to classify various types of compounds (e.g. alcohols, alkyl halides, amines) or reactive intermediates (e.g. alkyl radicals, carbocations). {{clear See also * Secondary (chemistry) * Tertiary (che ... * Secondary (chemistry) * Quaternary (chemistry) References {{Navbox stereochemistry Chemical nomenclature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tartaric Acid
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes but also in tamarinds, bananas, avocados, and citrus. Its salt (chemistry), salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of winemaking, fermentation. Potassium bitartrate is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E-numbers, E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic synthesis. Tartaric acid, an alpha-hydroxy-carboxylic acid, is diprotic acid, diprotic and aldaric acid, aldaric in acid characteristics and is a dihydroxyl derivative of succinic acid. History Tartaric acid has been known to winemakers for centuries. However, the chemical process for extraction was developed in 1769 by the Sweden, Swedish chemist Carl Wilhel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |