|
Mur Ligase
The bacterial cell wall provides strength and rigidity to counteract internal osmotic pressure, and protection against the environment. The peptidoglycan layer gives the cell wall its strength, and helps maintain the overall shape of the cell. The basic peptidoglycan structure of both Gram-positive and Gram-negative bacteria comprises a sheet of glycan chains connected by short cross-linking polypeptides. Biosynthesis of peptidoglycan is a multi-step (11-12 steps) process comprising three main stages: # formation of UDP-N-acetylmuramic acid (UDPMurNAc) from N-acetylglucosamine (GlcNAc). # addition of a short polypeptide chain to the UDPMurNAc. # addition of a second GlcNAc to the disaccharide- pentapeptide building block and transport of this unit through the cytoplasmic membrane and incorporation into the growing peptidoglycan layer. Stage two involves four key Mur ligase enzymes: MurC, MurD, MurEEC and MurFEC. These four Mur ligases are responsible for the successive additio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit the air, soil, water, Hot spring, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria play a vital role in many stages of the nutrient cycle by recycling nutrients and the nitrogen fixation, fixation of nitrogen from the Earth's atmosphere, atmosphere. The nutrient cycle includes the decomposition of cadaver, dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoplasmic Membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the cytoplasm, interior of a Cell (biology), cell from the extracellular space, outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane Membrane fluidity, fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane membrane transport, controls the movement of substances in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sequence Motif
In biology, a sequence motif is a nucleotide or amino-acid sequence pattern that is widespread and usually assumed to be related to biological function of the macromolecule. For example, an ''N''-glycosylation site motif can be defined as ''Asn, followed by anything but Pro, followed by either Ser or Thr, followed by anything but Pro residue''. Overview When a sequence motif appears in the exon of a gene, it may encode the " structural motif" of a protein; that is a stereotypical element of the overall structure of the protein. Nevertheless, motifs need not be associated with a distinctive secondary structure. " Noncoding" sequences are not translated into proteins, and nucleic acids with such motifs need not deviate from the typical shape (e.g. the "B-form" DNA double helix). Outside of gene exons, there exist regulatory sequence motifs and motifs within the " junk", such as satellite DNA. Some of these are believed to affect the shape of nucleic acids (see for example ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life. Amino acids can be classified according to the locations of the core structural functional groups ( alpha- , beta- , gamma- amino acids, etc.); other categories relate to polarity, ionization, and side-chain group type ( aliphatic, acyclic, aromatic, polar, etc.). In the form of proteins, amino-acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on Earth and its emergence. Amino acids are formally named by the IUPAC- IUBMB Joint Commi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrofolate Reductase
Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as an electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in one-carbon transfer chemistry. In humans, the DHFR enzyme is encoded by the ''DHFR'' gene. It is found in the q14.1 region of chromosome 5. There are two structural classes of DHFR, evolutionarily unrelated to each other. The former is usually just called DHFR and is found in bacterial chromosomes and animals. In bacteria, however, antibiotic pressure has caused this class to evolve different patterns of binding diaminoheterocyclic molecules, leading to many "types" named under this class, while mammalian ones remain highly similar. The latter (type II), represented by the plastid-encoded R67, is a tiny enzyme that works by forming a homotetramer. Function Dihydrofolate reductase converts dihydrofolate into tetrahydrofolate, a proton shuttle required for the de no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GTPase
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases. Functions GTPases function as molecular switches or timers in many fundamental cellular processes. Examples of these roles include: * Signal transduction in response to activation of cell surface receptors, including transmembrane receptors such as those mediating taste, smell and vision. * Protein biosynthesis (a.k.a. translation) at the ribosome. * Regulation of cell differentiation, proliferation, division and movement. * Translocation of proteins through membranes. * Transport of vesicles within the cell, and vesicle-mediated secretion and uptake, through GTPase control of vesicle coat assembly. GTPases are active when bound to GTP and inactive when bound to GDP. In the general ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATPase
ATPases (, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, ATP hydrolase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or the inverse reaction. This dephosphorylation reaction releases energy, which the enzyme (in most cases) harnesses to drive other chemical reactions that would not otherwise occur. This process is widely used in all known forms of life. Some such enzymes are integral membrane proteins (anchored within biological membranes), and move solutes across the membrane, typically against their concentration gradient. These are called transmembrane ATPases. Functions Transmembrane ATPases import metabolites necessary for cell metabolism and export toxins, wastes, and solutes that can hinder cellular processes. An important example is the sodium-potassium pump (Na+/K+ATPase) that maintains the cell membrane potential. Another example is the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATP-binding Domain
An ATP-binding motif is a 250-residue sequence within an ATP-binding protein’s primary structure. The binding motif is associated with a protein’s structure and/or function. ATP is a molecule of energy, and can be a coenzyme, involved in a number of biological reactions. ATP is proficient at interacting with other molecules through a binding site. The ATP binding site is the environment in which ATP catalytically actives the enzyme and, as a result, is hydrolyzed to ADP. The binding of ATP causes a conformational change to the enzyme it is interacting with. The genetic and functional similarity of such a motif demonstrates micro-evolution: proteins have co-opted the same binding sequence from other enzymes rather than developing them independently. ATP binding sites, which may be representative of an ATP binding motif, are present in many proteins which require an input of energy (from ATP), such sites as active membrane transporters, microtubule subunits, flagellum proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding (molecular)
Molecular binding is an attractive interaction between two molecules that results in a stable association in which the molecules are in close proximity to each other. It is formed when atoms or molecules bind together by sharing of electrons. It often, but not always, involves some chemical bonding. In some cases, the associations can be quite strong—for example, the protein streptavidin and the vitamin biotin have a dissociation constant (reflecting the ratio between bound and free biotin) on the order of 10−14—and so the reactions are effectively irreversible. The result of molecular binding is sometimes the formation of a molecular complex in which the attractive forces holding the components together are generally non-covalent, and thus are normally energetically weaker than covalent bonds. Molecular binding occurs in biological complexes (e.g., between pairs or sets of proteins, or between a protein and a small molecule ligand it binds) and also in abiologic chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rossmann Fold
The Rossmann fold is a tertiary fold found in proteins that bind nucleotides, such as enzyme cofactors FAD, NAD+, and NADP+. This fold is composed of alternating beta strands and alpha helical segments where the beta strands are hydrogen bonded to each other forming an extended beta sheet and the alpha helices surround both faces of the sheet to produce a three-layered sandwich. The classical Rossmann fold contains six beta strands whereas Rossmann-like folds, sometimes referred to as Rossmannoid folds, contain only five strands. The initial beta-alpha-beta (bab) fold is the most conserved segment of the Rossmann fold. The motif is named after Michael Rossmann who first noticed this structural motif in the enzyme lactate dehydrogenase in 1970 and who later observed that this was a frequently occurring motif in nucleotide binding proteins. Rossmann and Rossmannoid fold proteins are extremely common. They make up 20% of proteins with known structures in the Protein Data Bank, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sequence (biology)
A sequence in biology is the one-dimensional ordering of monomers, covalently linked within a biopolymer; it is also referred to as the primary structure of a biological macromolecule. While it can refer to many different molecules, the term sequence is most often used to refer to a DNA sequence or a protein sequence.https://www.nobelprize.org/uploads/2018/06/sanger-lecture.pdf See also * Dot plot (bioinformatics) * Sequence analysis In bioinformatics, sequence analysis is the process of subjecting a DNA, RNA or peptide sequence to any of a wide range of analytical methods to understand its features, function, structure, or evolution. It can be performed on the entire genome ... References Molecular biology {{molecular-biology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
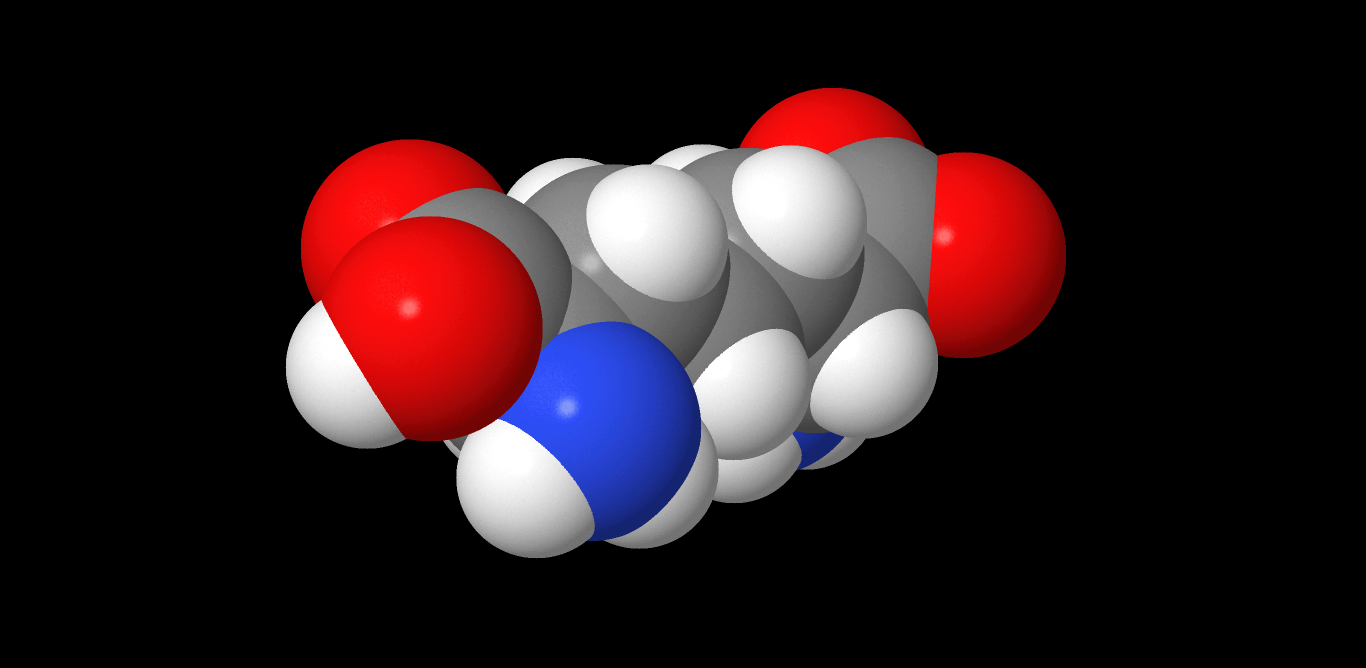
Diaminopimelate
Diaminopimelic acid (DAP) is an amino acid, representing an epsilon-carboxy derivative of lysine. ''meso''-α,ε-Diaminopimelic acid is the last intermediate in the biosynthesis of lysine and undergoes decarboxylation by diaminopimelate decarboxylase to give the final product. DAP is a characteristic of certain cell walls of some bacteria. DAP is often found in the peptide linkages of NAM- NAG chains that make up the cell wall of gram-negative bacteria. When provided, they exhibit normal growth. When in deficiency, they still grow but with the inability to make new cell wall peptidoglycan. This is also the attachment point for Braun's lipoprotein. See also * Aspartate-semialdehyde dehydrogenase, an enzyme involved in DAP synthesis * Peptidoglycan * Pimelic acid Pimelic acid is the organic compound with the formula HO2C(CH2)5CO2H. Pimelic acid is one unit longer than a related dicarboxylic acid, adipic acid, a precursor to many polyesters and polyamides. However compared ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |