|
Mischmetal
Mischmetal (from – "mixed metal") is an alloy of rare-earth elements. It is also called cerium mischmetal, or rare-earth mischmetal. A typical composition includes approximately 55% cerium, 25% lanthanum, and 15~18% neodymium, with traces of other rare earth metals totaling 95% lanthanides, plus 5% iron. Its most common use is in the pyrophoric ferrocerium "flint" ignition device of many lighters and torches. Because an alloy of only rare-earth elements would be too soft to give good sparks, it is blended with iron oxide and magnesium oxide to form a harder material known as ferrocerium. In chemical formulae it is commonly abbreviated as Mm, e.g. MmNi5. History Carl Auer von Welsbach was the discoverer of neodymium and praseodymium, and co-discoverer of lutetium. He was also the inventor of the gas mantle (using thorium) and of the rare-earth industry. After extracting thorium from monazite sand, many lanthanides remained, for which there was no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
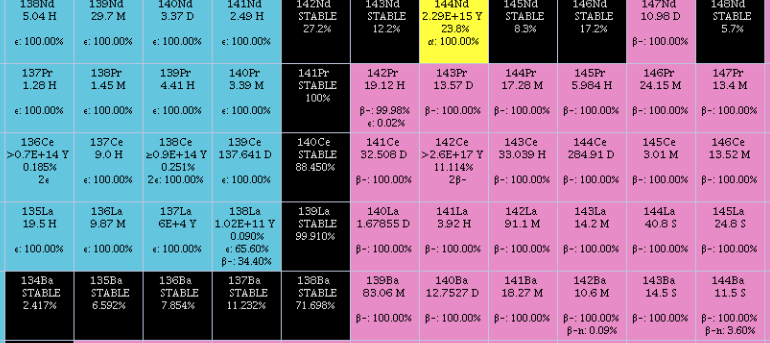
Cerium
Cerium is a chemical element; it has Chemical symbol, symbol Ce and atomic number 58. It is a hardness, soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the oxidation state of +3 characteristic of the series, it also has a stable +4 state that does not oxidize water. It is considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure. Despite always occurring in combination with the other rare-earth elements in minerals such as those of the monazite and bastnäsite groups, cerium is easy to extract from its ores, as it can be distinguished among the lanthanides by its unique ability to be oxidized to the +4 state in aqueous solution. It is the most common of the lanthanides, followed by neodymium, lanthanum, and praseodymium. Its estimated abundance of elements in Earth's crust, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferrocerium
Ferrocerium (also known in Europe as Auermetall) is a synthetic pyrophoric alloy of mischmetal (cerium, lanthanum, neodymium, other trace lanthanides and some iron – about 95% lanthanides and 5% iron) hardened by blending in oxides of iron and/or magnesium. When struck with a harder material, friction produces hot fragments that oxidize rapidly when exposed to the oxygen in the air, producing sparks that can reach temperatures of . The effect is due to the low ignition temperature of cerium, between . Ferrocerium has many commercial applications, such as the ignition source for lighters, strikers for gas welding and cutting torches, deoxidization in metallurgy, and ferrocerium rods. Because of ferrocerium's ability to ignite in adverse conditions, rods of ferrocerium (also called ferro rods, spark rods, and flint-spark-lighters) are commonly used as an emergency firelighting device in survival kits. The ferrocerium is referred to as a "flint" in this case, as bot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum
Lanthanum is a chemical element; it has symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, its usual oxidation state is +3, although some compounds are known with an oxidation state of +2. Lanthanum has no biological role in humans but is used by some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity. Lanthanum usually occurs together with cerium and the other rare earth elements. Lanthanum was first found by the Swedish chemist Carl Gustaf Mosander in 1839 as an impurity in cerium nitrate – hence the name ''lanthanum'', from the ancient Greek (), meaning 'to lie hidden'. Although ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexahydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are not inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain ''water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hydrate''. A colorful example is cobalt(II) chloride, which turns from blue to red upon mineral hydration, hydration, and can therefore be used as a water indicator. The notation "''hydrated compound''⋅''n''", where ''n'' is the number of water mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pronunciation of the word "chloride" is . Chloride salts such as sodium chloride are often soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Other examples of ionic chlorides include potassium chloride (), calcium chloride (), and ammonium chloride (). Examples of covalent chlorides include methyl chloride (), carbon tetrachloride (), sulfuryl chloride (), and monochloramine (). Electronic properties A chloride ion (diameter 167 pm) is much larger than a chlorine atom (diameter 99 pm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium Sulfate
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula Ba SO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs in nature as the mineral barite, which is the main commercial source of barium and materials prepared from it. Its opaque white appearance and its high density are exploited in its main applications.Holleman, A. F. and Wiberg, E. (2001) ''Inorganic Chemistry'', San Diego, CA. Academic Press, . Uses Drilling fluids About 80% of the world's barium sulfate production, mostly purified mineral, is consumed as a component of oil well drilling fluid. It increases the density of the fluid, increasing the hydrostatic pressure in the well and reducing the chance of a blowout. Radiocontrast agent Barium sulfate in suspension is often used medically as a radiocontrast agent for X-ray imaging and other diagnostic procedures. It is most often used in imaging of the GI tract during what is colloquially known as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radium
Radium is a chemical element; it has chemical symbol, symbol Ra and atomic number 88. It is the sixth element in alkaline earth metal, group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather than oxygen) upon exposure to air, forming a black surface layer of radium nitride (Ra3N2). All isotopes of radium are radioactive, the most stable isotope being radium-226 with a half-life of 1,600 years. When radium decays, it emits ionizing radiation as a by-product, which can excite fluorescent chemicals and cause radioluminescence. For this property, it was widely used in Self-luminous paint, self-luminous paints following its discovery. Of the Radionuclide, radioactive elements that occur in quantity, radium is considered particularly Toxicity, toxic, and it is Carcinogen, carcinogenic due to the radioactivity of both it and its immediate decay product radon as well as its tendency to B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare-earth
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set of 17 nearly indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. The term "rare-earth" is a misnomer because they are not actually scarce, but historically it took a long time to isolate these elements. They are relatively plentiful in the entire Earth's crust (cerium being the 25th-most-abundant element at 68 parts per million, more abundant than copper), but in practice they are spread thinly as trace impurities, so to obtain rare earths at usable purity requires processing enormous amounts of raw ore at great expense; thus the name "rare" earths. Scandium and yttrium are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basicity
In chemistry, there are three definitions in common use of the word "base": ''Arrhenius bases'', ''Brønsted bases'', and ''Lewis bases''. All definitions agree that bases are substances that react with acids, as originally proposed by Guillaume-François Rouelle, G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form hydroxide ions OH−. These ions can react with Hydron (chemistry), hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Calcium hydroxide, Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. They are slippery to the touch, can taste Taste#Bitterness, bitter and change the color of pH indicators (e.g., turn red litmus paper blue). In water, by altering the self-ionization of water, autoionization Chemical equi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly corrosive base (chemistry), base and alkali that decomposes lipids and proteins at ambient temperatures and at high concentrations may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the making of wood pulp and paper, tex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula . It is a colorless, odorless, and Viscosity, viscous liquid that is Miscibility, miscible with water. Pure sulfuric acid does not occur naturally due to its Dehydration reaction, strong affinity to water vapor; it is Hygroscopy, hygroscopic and readily absorbs water vapor from the Atmosphere of Earth, air. Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is releas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |