|
Metal–inorganic Framework
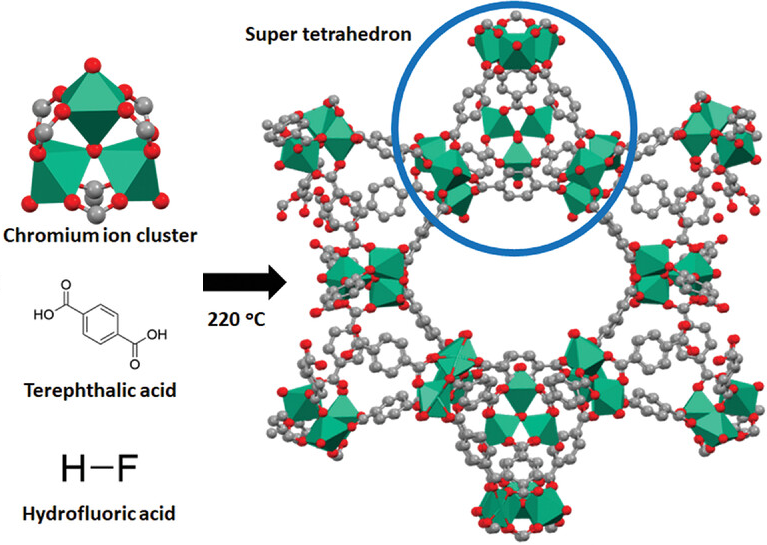
Metal–inorganic frameworks (MIFs) are a class of compounds consisting of metal ions or clusters coordinated to inorganic ligands to form one-, two-, or three-dimensional structures. They are a subclass of coordination polymers, with the special feature that they are often porous. They are inorganic counterpart of Metal–organic frameworks. __TOC__ History Millon's base which have been known since early 20th century, can be considered as MIFs. T.A.Shestimerova, A.V.Shevelkov Russ. Chem. Rev., 2018, 87 (1) 28 - 48 Linkers MIF with Borazocine linker was developed for hydrogen storage Several methods exist for storing hydrogen. These include mechanical approaches such as using high pressures and low temperatures, or employing chemical compounds that release H2 upon demand. While large amounts of hydrogen are produced by variou .... Cu2I2Se6 has Se6 linkers.Ding, N.; Armatas, G. S.; Kanatzidis, M. G. J. Am. Chem. Soc. 2010, 132, 6728. There are many MIFs with pnictogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against nonmetallic materials which do not. Metals are typically ductile (can be drawn into a wire) and malleable (can be shaped via hammering or pressing). A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polythiazyl, polymeric sulfur nitride. The general science of metals is called metallurgy, a subtopic of materials science; aspects of the electronic and thermal properties are also within the scope of condensed matter physics and solid-state chemistry, it is a multidisciplinary topic. In colloquial use materials such as steel alloys are referred to as metals, while others such as polymers, wood or ceramics are nonmetallic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ (potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− (chloride ion) and OH− (hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indicates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cluster Compound
Nanoclusters are atomically precise, crystalline materials most often existing on the 0-2 nanometer scale. They are often considered kinetically stable intermediates that form during the synthesis of comparatively larger materials such as semiconductor and metallic nanocrystals. The majority of research conducted to study nanoclusters has focused on characterizing their crystal structures and understanding their role in the nucleation and growth mechanisms of larger materials. Materials can be categorized into three different regimes, namely bulk, nanoparticles and nanoclusters. Bulk metals are electrical conductors and good optical reflectors and metal nanoparticles display intense colors due to surface plasmon resonance. However, when the size of metal nanoclusters is further reduced to form a nanocluster, the electronic band structure, band structure becomes discontinuous and breaks down into discrete energy levels, somewhat similar to the energy levels of molecules. This gi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep Mantle (geology), mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salt (chemistry), salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within life, living things. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligands
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Polymer
Coordination may refer to: * Coordination (linguistics), a compound grammatical construction * Coordination complex, consisting of a central atom or ion and a surrounding array of bound molecules or ions ** A chemical reaction to form a coordination complex * Coordination number or ligancy of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it * Language coordination, the tendency of people to mimic the language of others * Coordination (political culture), a Utopian form of political regime * Motor coordination In physiology, motor coordination is the orchestrated movement of multiple body parts as required to accomplish intended actions, like walking. This coordination is achieved by adjusting kinematic and kinetic parameters associated with each bo ..., in animal motion * '' Gleichschaltung'' the process of Nazification in Germany after 1933, often translated as "coordination" See also * Coordinate (other) * Coordin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porous
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure the "accessible void", the total amount of void space accessible from the surface (cf. closed-cell foam). There are many ways to test porosity in a substance or part, such as industrial CT scanning. The term porosity is used in multiple fields including pharmaceutics, ceramics, metallurgy, materials, manufacturing, petrophysics, hydrology, earth sciences, soil mechanics, rock mechanics, and engineering. Void fraction in two-phase flow In gas-liquid two-phase flow, the void fraction is defined as the fraction of the flow-channel volume that is occupied by the gas phase or, alternatively, as the fraction of the cross-sectional area of the channel that is occupied by the gas phase. Void fraction usually varies from location to loca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal–organic Framework
Metal–organic frameworks (MOFs) are a class of porous polymers consisting of metal cluster compound, clusters (also known as Secondary Building Units - SBUs) coordinated to organic compound, organic ligands to form one-, two- or three-dimensional structures. The organic ligands included are sometimes referred to as "struts" or "linkers", one example being terephthalic acid, 1,4-benzenedicarboxylic acid (BDC). MOFs are classified as reticular materials. More formally, a metal–organic framework is a potentially porous extended structure made from metal ions and organic linkers. An extended structure is a structure whose sub-units occur in a constant ratio and are arranged in a repeating pattern. MOFs are a subclass of coordination networks, which is a coordination compound extending, through repeating coordination entities, in one dimension, but with cross-links between two or more individual chains, loops, or spiro-links, or a coordination compound extending through repeating c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Millon's Base
Mercuric amidochloride is an inorganic compound with the formula . Preparation and properties It arises from the reaction of mercury(II) chloride and ammonia (Calomel reaction), where the resulting mercuric amidochloride is highly insoluble. It forms white crystals in the shape of small prisms. It tastes earthy and metallic, but is a deadly poison and should not be ingested. At the molecular level, it organizes as a zig-zag 1-dimensional polymer with chloride counterions. It is stable in air, but darkens on exposure to light. It does not melt, even at dull red heat, instead subliming and decomposing to gaseous mercury, hydrogen chloride, and nitrogen oxides. Consequently sealed containers with this chemical may explode when heated. The substance is a deadly poison, although not a carcinogen. It is toxic unto lethality by inhalation, ingestion or dermal absorption. In lesser cases, it may instead cause dermatitis and skin lesions or corrode the mucous membranes. If i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borazocine
Borazocine is a polar inorganic compound with the chemical formula B4H8N4. In this cyclic compound, the four BH units and four NH units alternate. Related compounds Borazine is a six-membered aromatic ring with three boron atoms and three nitrogen atoms. See also * 1,2-Dihydro-1,2-azaborine * Cyclooctatetraene 1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of ... References {{Reflist Nitrogen heterocycles Boron heterocycles Eight-membered rings ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Storage
Several methods exist for storing hydrogen. These include mechanical approaches such as using high pressures and low temperatures, or employing chemical compounds that release H2 upon demand. While large amounts of hydrogen are produced by various industries, it is mostly consumed at the site of production, notably for the synthesis of ammonia. For many years hydrogen has been stored as compressed gas or cryogenic liquid, and transported as such in cylinders, tubes, and cryogenic tanks for use in industry or as propellant in space programs. The overarching challenge is the very low boiling point of H2: it boils around 20.268 K (−252.882 °C or −423.188 °F). Achieving such low temperatures requires expending significant energy. Although molecular hydrogen has very high energy density on a mass basis, partly because of its low molecular weight, as a gas at ambient conditions it has very low energy density by volume. If it is to be used as fuel stored on board a vehic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
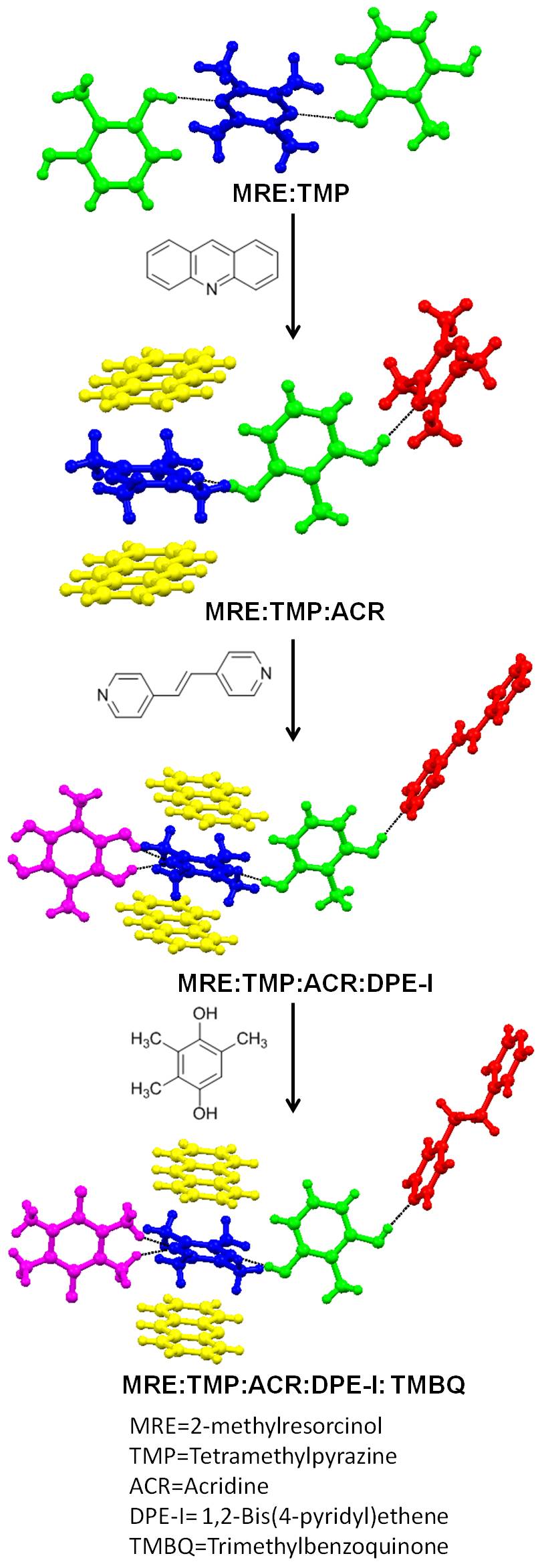
Crystal Engineering
Crystal engineering studies the design and synthesis of solid-state structures with desired properties through deliberate control of Intermolecular force, intermolecular interactions. It is an Interdisciplinarity, interdisciplinary academic field, bridging solid-state and supramolecular chemistry. The main engineering strategies currently in use are hydrogen bond, hydrogen- and halogen bonding and coordination bonding. These may be understood with key concepts such as the supramolecular synthon and the secondary building unit. History of term The term 'crystal engineering' was first used in 1955 by R. Pepinsky but the starting point is often credited to Gerhard Schmidt in connection with photodimerization reactions in crystalline cinnamic acids. Since this initial use, the meaning of the term has broadened considerably to include many aspects of solid state supramolecular chemistry. A useful modern definition is that provided by Gautam Radhakrishna Desiraju, Gautam Desiraju, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |