crystal engineering on:
[Wikipedia]
[Google]
[Amazon]
Crystal engineering studies the design and synthesis of solid-state structures with desired properties through deliberate control of
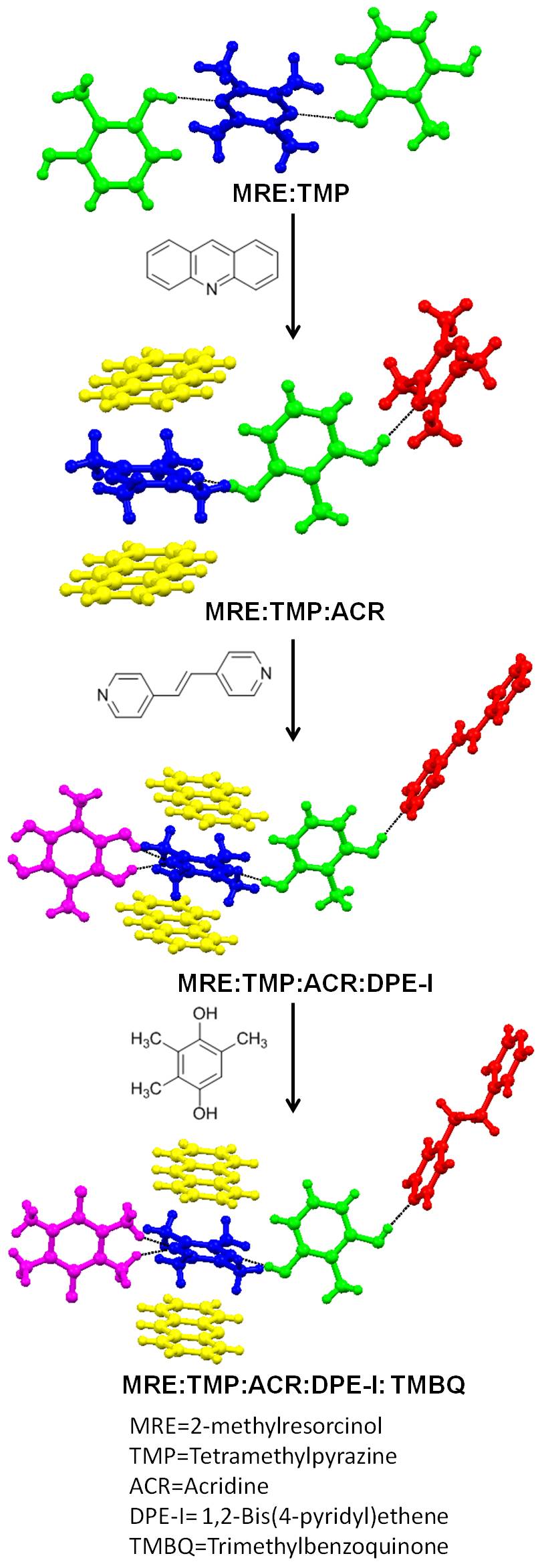 The intentional synthesis of
The intentional synthesis of
 The design of crystal structures with desired properties is the ultimate goal of crystal engineering. Crystal engineering principles have been applied to the design of non-linear optical materials, especially those with second harmonic generation (SHG) properties. Using supramolecular synthons, supramolecular gels have been designed.
The design of crystal structures with desired properties is the ultimate goal of crystal engineering. Crystal engineering principles have been applied to the design of non-linear optical materials, especially those with second harmonic generation (SHG) properties. Using supramolecular synthons, supramolecular gels have been designed.

 Designing a crystalline material with targeted properties requires an understanding of the material's molecular and crystal features in relation to its
Designing a crystalline material with targeted properties requires an understanding of the material's molecular and crystal features in relation to its
 A supramolecular synthon is a pair of molecules that form relatively strong intermolecular interactions in the early phases of
A supramolecular synthon is a pair of molecules that form relatively strong intermolecular interactions in the early phases of
Crystal Growth and DesignCrystEngCommActa Crystallographica Section BCambridge Structural Database
{{Authority control Materials science Chemical product engineering Solid-state chemistry Supramolecular chemistry Crystallography
intermolecular interactions
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
. It is an interdisciplinary
Interdisciplinarity or interdisciplinary studies involves the combination of multiple academic disciplines into one activity (e.g., a research project). It draws knowledge from several fields such as sociology, anthropology, psychology, economi ...
academic field
An academic discipline or academic field is a subdivision of knowledge that is taught and researched at the college or university level. Disciplines are defined (in part) and recognized by the academic journals in which research is published, a ...
, bridging solid-state and supramolecular chemistry.
The main engineering strategies currently in use are hydrogen- and halogen bond
In chemistry, a halogen bond (XB or HaB) occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecul ...
ing and coordination bonding. These may be understood with key concepts such as the supramolecular synthon
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 1988 ...
and the secondary building unit.
History of term
The term 'crystal engineering' was first used in 1955 by R. Pepinsky but the starting point is often credited to Gerhard Schmidt in connection with photodimerization reactions in crystallinecinnamic acid
Cinnamic acid is an organic compound with the formula phenyl, C6H5-CH=CH-Carboxylic acid, COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxy ...
s. Since this initial use, the meaning of the term has broadened considerably to include many aspects of solid state supramolecular chemistry
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from w ...
. A useful modern definition is that provided by Gautam Desiraju, who in 1988 defined crystal engineering as "the understanding of intermolecular interactions in the context of crystal packing and the utilization of such understanding in the design of new solids with desired physical and chemical properties." Since many of the bulk properties of molecular materials are dictated by the manner in which the molecules are ordered in the solid state, it is clear that an ability to control this ordering would afford control over these properties.
Non-covalent control of structure
Crystal engineering relies on noncovalent bonding to achieve the organization of molecules and ions in the solid state. Much of the initial work on purelyorganic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ...
systems focused on the use of hydrogen bonds, although coordination and halogen bond
In chemistry, a halogen bond (XB or HaB) occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecul ...
s provide additional control in crystal design.
Molecular self-assembly
In chemistry and materials science, molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly: intermolecular and intramolec ...
is at the heart of crystal engineering, and it typically involves an interaction between complementary hydrogen bonding faces or a metal and a ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
. "Supramolecular synthons" are building blocks that are common to many structures and hence can be used to order specific groups in the solid state.
Design of multi-component crystals
 The intentional synthesis of
The intentional synthesis of cocrystal
In materials science (specifically crystallography), cocrystals are "solids that are crystalline, single- phase materials composed of two or more different molecular or ionic compounds generally in a stoichiometric ratio which are neither solva ...
s is most often achieved with strong heteromolecular interactions. The main relevance of multi-component crystals is focused upon designing pharmaceutical cocrystals. Pharmaceutical cocrystals are generally composed of one API (Active Pharmaceutical Ingredient
An active ingredient is any ingredient that provides biological activity, biologically active or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure or any function of the body of ...
) with other molecular substances that are considered safe according to the guidelines provided by WHO (World Health Organization
The World Health Organization (WHO) is a list of specialized agencies of the United Nations, specialized agency of the United Nations which coordinates responses to international public health issues and emergencies. It is headquartered in Gen ...
). Various properties (such as solubility, bioavailability, permeability) of an API can be modulated through the formation of pharmaceutical cocrystals.
In two dimensions
2D architectures (i.e., molecularly thick architectures) is a branch of crystal engineering. The formation (often referred asmolecular self-assembly
In chemistry and materials science, molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly: intermolecular and intramolec ...
depending on its deposition process) of such architectures lies in the use of solid interfaces to create adsorbed monolayers. Such monolayers may feature spatial crystallinity. However the dynamic and wide range of monolayer morphologies ranging from amorphous to network structures have made of the term (2D) supramolecular engineering a more accurate term. Specifically, supramolecular engineering refers to "(The) design (of) molecular units in such way that a predictable structure is obtained" or as "the design, synthesis and self-assembly of well defined molecular modules into tailor-made supramolecular architectures".
Scanning probe microscopic techniques enable visualization of two dimensional assemblies.
Polymorphism
Polymorphism, the phenomenon wherein the same chemical compound exists in more than one crystal forms, is relevant commercially because polymorphic forms of drugs may be entitled to independent patent protection. The importance of crystal engineering to the pharmaceutical industry is expected to grow exponentially. Polymorphism arises due to the competition between kinetic and thermodynamic factors during crystallization. While long-range strong intermolecular interactions dictate the formation of kinetic crystals, the close packing of molecules generally drives the thermodynamic outcome. Understanding this dichotomy between the kinetics and thermodynamics constitutes the focus of research related to the polymorphism. In organic molecules, three types of polymorphism are mainly observed. Packing polymorphism arises when molecules pack in different ways to give different structures. Conformational polymorphism, on the other hand is mostly seen in flexible molecules where molecules have multiple conformational possibilities within a small energy window. As a result, multiple crystal structures can be obtained with the same molecule but in different conformations. The rarest form of polymorphism arises from the differences in the primary synthon and this type of polymorphism is called as synthon polymorphism.Crystal structure prediction
Crystal structure prediction Crystal structure prediction (CSP) is the calculation of the crystal structures of solids from first principles. Reliable methods of predicting the crystal structure of a compound, based only on its composition, has been a goal of the physical scien ...
(CSP) is a computational approach to generate energetically feasible crystal structures (with corresponding space group and positional parameters) from a given molecular structure. The CSP exercise is considered most challenging as "experimental" crystal structures are very often kinetic structures and therefore are very difficult to predict. In this regard, many protocols have been proposed and are tested through several blind tests organized by CCDC since 2002. A major advance in the CSP happened in 2007 while a hybrid method based on tailor made force fields and density functional theory (DFT) was introduced. In the first step, this method employs tailor made force fields to decide upon the ranking of the structures followed by a dispersion corrected DFT method to calculate the lattice energies precisely.
Apart from the ability of predicting crystal structures, CSP also gives computed energy landscapes of crystal structures where many structures lie within a narrow energy window. This kind of computed landscapes lend insights into the study on polymorphism, design of new structures and also help to design crystallization experiments.
Property design
 The design of crystal structures with desired properties is the ultimate goal of crystal engineering. Crystal engineering principles have been applied to the design of non-linear optical materials, especially those with second harmonic generation (SHG) properties. Using supramolecular synthons, supramolecular gels have been designed.
The design of crystal structures with desired properties is the ultimate goal of crystal engineering. Crystal engineering principles have been applied to the design of non-linear optical materials, especially those with second harmonic generation (SHG) properties. Using supramolecular synthons, supramolecular gels have been designed.
Mechanical properties of crystalline materials

 Designing a crystalline material with targeted properties requires an understanding of the material's molecular and crystal features in relation to its
Designing a crystalline material with targeted properties requires an understanding of the material's molecular and crystal features in relation to its mechanical properties
A material property is an intensive property of a material, i.e., a physical property or chemical property that does not depend on the amount of the material. These quantitative properties may be used as a metric by which the benefits of one mate ...
. Four mechanical properties are of interest for crystalline materials: plasticity
Plasticity may refer to:
Science
* Plasticity (physics), in engineering and physics, the propensity of a solid material to undergo permanent deformation under load
* Behavioral plasticity, change in an organism's behavior in response to exposur ...
, elasticity, brittleness
A material is brittle if, when subjected to stress (physics), stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of h ...
, and shear strength
In engineering, shear strength is the strength of a material or component against the type of yield or structural failure when the material or component fails in shear. A shear load is a force that tends to produce a sliding failure on a mater ...
).
Intermolecular interactions
Manipulation of the intermolecular interaction network is a means for controlling bulk properties. Duringcrystallization
Crystallization is a process that leads to solids with highly organized Atom, atoms or Molecule, molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regu ...
, intermolecular interactions
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
form according to an electrostatic hierarchy. Strong hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
s are the primary director for crystal organization.
Crystal architecture
Typically, the strongestintermolecular interactions
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
form the molecular layers or columns and the weakest intermolecular interactions
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
form the slip plane. For example, long chains or layers of acetaminophen
Paracetamol, or acetaminophen, is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely available over-the-counter drug sold under various brand names, including Tylenol and Panadol.
Parac ...
molecules form due to the hydrogen bond donors and acceptors that flank the benzene ring. The weaker interactions between the chains or layers of acetaminophen required less energy to break than the hydrogen bonds. As a result, a slip plane is formed.
 A supramolecular synthon is a pair of molecules that form relatively strong intermolecular interactions in the early phases of
A supramolecular synthon is a pair of molecules that form relatively strong intermolecular interactions in the early phases of crystallization
Crystallization is a process that leads to solids with highly organized Atom, atoms or Molecule, molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regu ...
; these molecule pairs are the basic structural motif found in a crystal lattice
Lattice may refer to:
Arts and design
* Latticework, an ornamental criss-crossed framework, an arrangement of crossing laths or other thin strips of material
* Lattice (music), an organized grid model of pitch ratios
* Lattice (pastry), an or ...
.
Defects or imperfections
Lattice defects, such as point defects, tilt boundaries, or dislocations, create imperfections in crystal architecture and topology. Any disruption to thecrystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
alters the mechanism or degree of molecular movement, thereby changing the mechanical properties of the material. Examples of point imperfections include vacancies, substitutional impurities, interstitial impurities, Frenkel’s defects, and Schottky’s defects. Examples of line imperfections include edge
Edge or EDGE may refer to:
Technology Computing
* Edge computing, a network load-balancing system
* Edge device, an entry point to a computer network
* Adobe Edge, a graphical development application
* Microsoft Edge, a web browser developed by ...
and screw dislocations.
Assessing Crystal Structure
Crystallographic methods, such asX-ray diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. ...
, are used to elucidate the crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
of a material by quantifying distances between atoms. The X-ray diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. ...
technique relies on a particular crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
creating a unique pattern after X-rays are diffracted through the crystal lattice
Lattice may refer to:
Arts and design
* Latticework, an ornamental criss-crossed framework, an arrangement of crossing laths or other thin strips of material
* Lattice (music), an organized grid model of pitch ratios
* Lattice (pastry), an or ...
. Microscopic methods, such as optical
Optics is the branch of physics that studies the behaviour and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behaviour of visible, ultravio ...
, electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
, field ion, and scanning tunneling microscopy
A scanning tunneling microscope (STM) is a type of scanning probe microscope used for imaging surfaces at the atomic level. Its development in 1981 earned its inventors, Gerd Binnig and Heinrich Rohrer, then at IBM Zürich, the Nobel Prize in ...
, can be used to visualize the microstructure
Microstructure is the very small scale structure of a material, defined as the structure of a prepared surface of material as revealed by an optical microscope above 25× magnification. The microstructure of a material (such as metals, polymer ...
, imperfections, or dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to sli ...
s of a material. Ultimately, these methods elaborate on the growth and assembly of crystallite
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains.
Bacillite is a type of crystallite. It is rodlike with parallel Wikt:longulite ...
s during crystallization
Crystallization is a process that leads to solids with highly organized Atom, atoms or Molecule, molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regu ...
, which can be used to rationalize the movement of crystallite
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains.
Bacillite is a type of crystallite. It is rodlike with parallel Wikt:longulite ...
s in response to an applied load. Calorimetric methods, such as differential scanning calorimetry
Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and re ...
, use induce phase transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...
s in order to quantify the associated changes in enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
, entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
, and Gibb's free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of work, other than pressure–volume work, that may be performed by a ther ...
. The melting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which inc ...
and fusion phase transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...
s are dependent on the lattice energy
In chemistry, the lattice energy is the energy change (released) upon formation of one mole of a crystalline compound from its infinitely separated constituents, which are assumed to initially be in the gaseous state at 0 K. It is a measure of ...
of the crystalline material, which can be used to determine percent crystallinity
Crystallinity refers to the degree of structural order in a solid. In a crystal, the atoms or molecules are arranged in a regular, periodic manner. The degree of crystallinity has a large influence on hardness, density, transparency and diffusi ...
of the sample. Raman spectroscopy is a method that uses light scattering to interact with bonds in a sample. This technique provides information about chemical bonds, intermolecular interactions, and crystallinity.
Assessing mechanical properties
Nanoindentation
Nanoindentation, also called instrumented indentation testing, is a variety of indentation hardness tests applied to small volumes. Indentation is perhaps the most commonly applied means of testing the mechanical properties of materials. The nanoi ...
is a standard and widely-accepted method for measuring mechanical properties within the crystal engineering field.S. Varughese, M. S. R. N. Kiran, U. Ramamurty and G. R. Desiraju, ''Nanoindentation in Crystal Engineering: Quantifying Mechanical Properties of Molecular Crystals'', ''Angew. Chem. Int. Ed.'' 2013, ''52'', 2701-2712. The method quantifies hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to plastic deformation, such as an indentation (over an area) or a scratch (linear), induced mechanically either by Pressing (metalworking), pressing or abrasion ...
, elasticity, packing anisotropy
Anisotropy () is the structural property of non-uniformity in different directions, as opposed to isotropy. An anisotropic object or pattern has properties that differ according to direction of measurement. For example, many materials exhibit ve ...
, and polymorphism of a crystalline material. Hirshfeld surfaces are visual models of electron density
Electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typical ...
at a specific isosurface that aid in visualizing and quantifying intermolecular interactions
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
. An advantage to using Hirshfeld surfaces in crystal engineering is that these surface maps are embedded with information about a molecular and its neighbors. The insight into molecular neighbors can be applied to assessment or prediction of molecular properties. An emerging method for topography and slip plane analysis using energy frameworks, which are models of crystal packing that depict interaction energies as pillars or beams.
See also
*Coordination polymers
Coordination may refer to:
* Coordination (linguistics), a compound grammatical construction
* Coordination complex, consisting of a central atom or ion and a surrounding array of bound molecules or ions
** A chemical reaction to form a coordinati ...
* crystal nets (periodic graphs)
* Crystallography
Crystallography is the branch of science devoted to the study of molecular and crystalline structure and properties. The word ''crystallography'' is derived from the Ancient Greek word (; "clear ice, rock-crystal"), and (; "to write"). In J ...
* Laser-heated pedestal growth
Laser-heated pedestal growth (LHPG) or laser floating zone (LFZ) is a crystal growth technique. A narrow region of a crystal is melted with a powerful Carbon-dioxide laser, CO2 or Nd:YAG laser. The laser and hence the zone melting, floating zone, i ...
* CrystEngComm
''CrystEngComm'' is a peer-reviewed online-only scientific journal publishing original research and review articles on all aspects of crystal engineering including properties, polymorphism, target materials, and crystalline nanomaterials. It i ...
* '' Crystal Growth & Design''
* CrystEngCommunity CrystEngCommunity is a virtual web community for people working in the field of crystal engineering. The website is owned by the Royal Society of Chemistry (RSC).
CrystEngCommunity has links to the main international research groups working in cr ...
* Hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
* Molecular design software Molecular design software is notable software for molecular modeling, that provides special support for developing molecular models ''de novo''.
In contrast to the usual molecular modeling programs, such as for molecular dynamics and quantum chemi ...
* Supramolecular chemistry
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from w ...
* Self-assembly
Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the ...
* Molecular self-assembly
In chemistry and materials science, molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly: intermolecular and intramolec ...
References
External links
Crystal Growth and Design
{{Authority control Materials science Chemical product engineering Solid-state chemistry Supramolecular chemistry Crystallography