|
Laser-heated Pedestal Growth
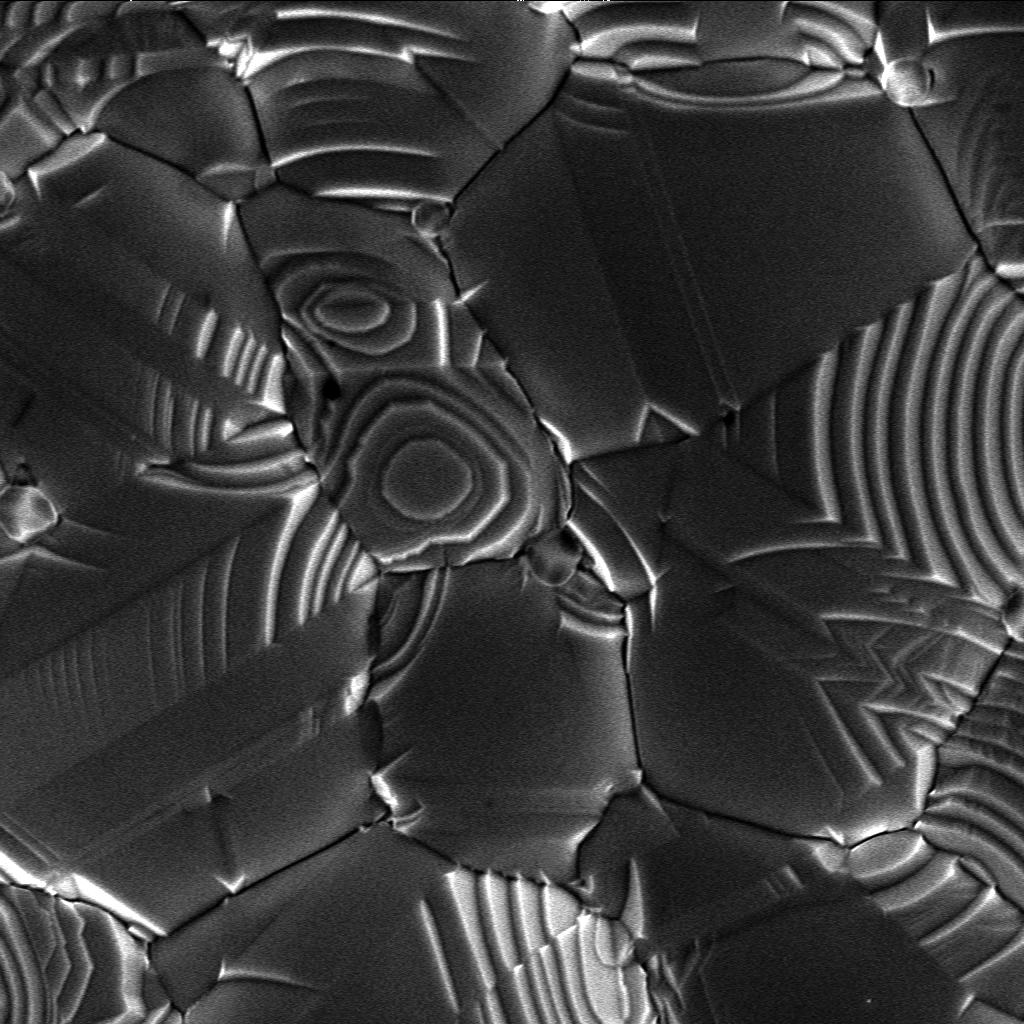
Laser-heated pedestal growth (LHPG) or laser floating zone (LFZ) is a crystal growth technique. A narrow region of a crystal is melted with a powerful Carbon-dioxide laser, CO2 or Nd:YAG laser. The laser and hence the zone melting, floating zone, is moved along the crystal. The molten region melts impure solid at its forward edge and leaves a wake of purer material solidified behind it. This technique for growing crystals from the melt (liquid/solid phase transition) is used in materials research. Advantages The main advantages of this technique are the high pulling rates (60 times greater than the conventional Czochralski technique) and the possibility of growing materials with very high melting points. In addition, LHPG is a crucible-free technique, which allows single crystals to be grown with high purity and low stress. The geometric shape of the crystals (the technique can produce small diameters), and the low production cost, make the single-crystal fibers (SCF) produced by L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Growth
Crystal growth is a major stage of a crystallization, crystallization process, and consists of the addition of new atoms, ions, or polymer strings into the characteristic arrangement of the crystalline lattice. The growth typically follows an initial stage of either homogeneous or heterogeneous (surface catalyzed) nucleation, unless a "seed" crystal, purposely added to start the growth, was already present. The action of crystal growth yields a crystalline solid whose atoms or molecules are close packed, with fixed positions in space relative to each other. The crystalline states of matter, state of matter is characterized by a distinct structural rigidity and very high resistance to Plastic deformation in solids, deformation (i.e. changes of shape and/or volume). Most crystalline solids have high values both of Young's modulus and of the shear modulus of elasticity (physics), elasticity. This contrasts with most liquids or fluids, which have a low shear modulus, and typically exh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Convection
Convection is single or Multiphase flow, multiphase fluid flow that occurs Spontaneous process, spontaneously through the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the convection is unspecified, convection due to the effects of thermal expansion and buoyancy can be assumed. Convection may also take place in soft solids or mixtures where particles can flow. Convective flow may be Transient state, transient (such as when a Multiphasic liquid, multiphase mixture of oil and water separates) or steady state (see convection cell). The convection may be due to Gravity, gravitational, Electromagnetism, electromagnetic or Fictitious force, fictitious body forces. Convection (heat transfer), Heat transfer by natural convection plays a role in the structure of Earth's atmosphere, its oceans, and its Earth's mantle, mantle. Discrete convective cells in the atmosphere can be identified by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recrystallization (metallurgy)
In materials science, recrystallization is a process by which deformed grains are replaced by a new set of defect-free grains that nucleate and grow until the original grains have been entirely consumed. Recrystallization is usually accompanied by a reduction in the strength and hardness of a material and a simultaneous increase in the ductility. Thus, the process may be introduced as a deliberate step in metals processing or may be an undesirable byproduct of another processing step. The most important industrial uses are softening of metals previously hardened or rendered brittle by cold work, and control of the grain structure in the final product. Recrystallization temperature is typically 0.3–0.4 times the melting point for pure metals and 0.5 times for alloys. Definition Recrystallization is defined as the process in which grains of a crystal structure come in a new structure or new crystal shape. A precise definition of recrystallization is difficult to state as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protocrystalline
A protocrystalline phase is a distinct phase occurring during crystal growth, which evolves into a microcrystalline form. The term is typically associated with silicon films in optical applications such as solar cells. Applications Silicon solar cells Amorphous silicon (a-Si) is a popular solar cell material owing to its low cost and ease of production. Owing to its disordered structure ( Urbach tail), its absorption extends to the energies below the band gap, resulting in a wide-range spectral response; however, it has a relatively low solar cell efficiency. Protocrystalline Si (pc-Si:H) also has a relatively low absorption near the band gap, owing to its more ordered crystalline structure. Thus, protocrystalline and amorphous silicon can be combined in a tandem solar cell, where the top thin layer of a-Si:H absorbs short-wavelength light whereas the underlying protocrystalline silicon layer absorbs the longer wavelengths See also *Amorphous silicon *Crystallite * Multijunc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleation
In thermodynamics, nucleation is the first step in the formation of either a new Phase (matter), thermodynamic phase or Crystal structure, structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled (at atmospheric pressure) significantly below 0°C, it will tend to Freezing, freeze into ice, but volumes of water cooled only a few degrees below 0°C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay. Nucleation is a common mechanism which generates first-order phase transitions, and it is the start of the process of forming a new thermodynamic phase. In contrast, new phas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Micro-pulling-down
The micro-pulling-down (μ-PD) method is a crystal growth technique based on continuous transport of the melted substance through micro-channel(s) made in a crucible bottom. Continuous solidification of the melt is progressed on a liquid/solid interface positioned under the crucible. In a steady state, both the melt and the crystal are pulled-down with a constant (but generally different) velocity. Many different types of crystal are grown by this technique, including Y3Al5O12, Si, Si-Ge, LiNbO3, α-Al2O3, Y2O3, Sc2O3, LiF, CaF2, BaF2, etc. Crystal growth routine Standard routine procedure used in the growth of most of μ-PD crystals is well developed. The general stages of the growths include: * Charging of the crucible with starting materials (mixture of powders) * Heating of the crucible until starting materials in the crucible are completely melted * Upward displacement of the seed until its contact with the meniscus or crucible * Formation of the meniscus and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fractional Crystallization (chemistry)
{{crystallization In chemistry, fractional crystallization is a stage-wise separation technique that relies on the liquid–solid phase change. This technique fractionates via differences in crystallization temperature and enables the purification of multi-component mixtures, as long as none of the constituents can act as solvents to the others. Due to the high selectivity of the solid–liquid equilibrium, very high purities can be achieved for the selected component. Principle of separation The crystallization process starts with the partial freezing of the initial liquid mixture by slowly decreasing its temperature. The frozen solid phase subsequently has a different composition than the remaining liquid. This is the fundamental physical principle behind the melt fractionating process and quite comparable to distillation, which operates between a liquid and the gas phase. The crystals will grow on a cooled surface or alternatively as a suspension in the liquid. The heat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallization (engineering Aspects)
Crystallization is a process that leads to solids with highly organized atoms or molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regular organization. Crystallization can occur by various routes including precipitation from solution, freezing of a liquid, or deposition from a gas. Attributes of the resulting crystal can depend largely on factors such as temperature, air pressure, cooling rate, or solute concentration. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles form layers at the crystal's surface and lodge themselves into open inconsistencies such as pores, cracks, etc. Crystallizatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallization
Crystallization is a process that leads to solids with highly organized Atom, atoms or Molecule, molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regular organization. Crystallization can occur by various routes including Precipitation (chemistry), precipitation from solution, freezing of a liquid, or Deposition (phase transition), deposition from a gas. Attributes of the resulting crystal can depend largely on factors such as temperature, air pressure, cooling rate, or Solution (chemistry), solute concentration. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a Supercooling, supercooled liquid or a supersaturation, supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallite
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains. Bacillite is a type of crystallite. It is rodlike with parallel Wikt:longulite , longulites. Structure The orientation of crystallites can be random with no preferred direction, called random Texture (chemistry), texture, or directed, possibly due to growth and processing conditions. While the structure of a single crystal is highly ordered and its crystal lattice, lattice is continuous and unbroken, amorphous solid, amorphous materials, such as glass and many polymers, are non-crystalline and do not display any structures, as their constituents are not arranged in an ordered manner. Polycrystalline structures and paracrystalline phases are in between these two extremes. Polycrystalline materials, or polycrystals, are solids that are composed of many crystallites of varying size and orientation. Most materials are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter. The smallest group of particles in a material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of principal axes/edges, of the unit cell and angles between them are lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of a crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name originates from Spanish language, Spanish , a diminutive of "silver". Platinum is a member of the platinum group of elements and group 10 element, group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the Abundance of elements in Earth's crust, rarer elements in Earth's crust, with an average abundance of approximately 5 microgram, μg/kg, making platinum about 30 times rarer than gold. It occurs in some nickel and copper ores along with some Native element mineral, native deposits, with 90% of current production from deposits across Russia's Ural Mountains, Colombia, the Sudbury Basin, Sudbury basin of Canada, and a large reserve in South Africa. Because of its scarcity in Earth's crust, only a f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |