|
Max Joseph Von Pettenkofer
Max Joseph Pettenkofer, ennobled in 1883 as Max Joseph von Pettenkofer (3 December 1818 – 10 February 1901) was a Bavarian chemist and hygienist. He is known for his work in practical hygiene, as an apostle of good water, fresh air and proper sewage disposal. He was further known as an anti-contagionist, a school of thought, named later on, that did not believe in the then novel concept that bacteria were the main cause of disease. In particular he argued in favor of a variety of conditions collectively contributing to the incidence of disease including: personal state of health, the fermentation of environmental ground water, and also the germ in question. He was most well known for his establishment of hygiene as an experimental science and also was a strong proponent for the founding of hygiene institutes in Germany. His work served as an example which other institutes around the world emulated. Early life and education Pettenkofer was born in Lichtenheim, near Neuburg an d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Max Von Pettenkofer5
Max or MAX may refer to: Animals * Max (American dog) (1983–2013), at one time purported to be the world's oldest living dog * Max (British dog), the first pet dog to win the PDSA Order of Merit (animal equivalent of the OBE) * Max (gorilla) (1971–2004), a western lowland gorilla at the Johannesburg Zoo who was shot by a criminal in 1997 Brands and enterprises * Australian Max Beer * Max Hamburgers, a fast-food corporation * MAX Index, a Hungarian domestic government bond index * Max Fashion, an Indian clothing brand Computing * MAX (operating system), a Spanish-language Linux version * Max (software), a music programming language * MAX Machine * Multimedia Acceleration eXtensions, extensions for HP PA-RISC Films * ''Max'' (1994 film), a Canadian film by Charles Wilkinson * ''Max'' (2002 film), a film about Adolf Hitler * ''Max'' (2015 film), an American war drama film * ''Max'' (2024 film), an Indian Kannada language film by Vijay Karthikeyaa Games * '' Dancing Stage ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
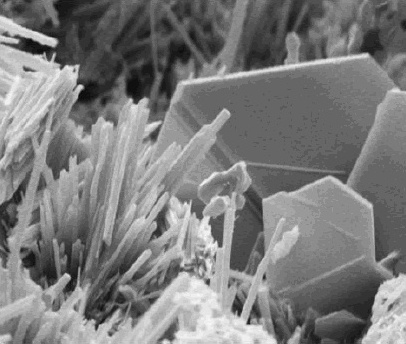
Oxalic Acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula , also written as or or . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name is derived from early investigators who isolated oxalic acid from flowering plants of the genus '' Oxalis'', commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous. Oxalic acid is a much stronger acid than acetic acid. It is a reducing agent and its conjugate bases hydrogen oxalate () and oxalate () are chelating agents for metal cations. It is used as a cleaning agent, especially for the removal of rust, because it forms a water-soluble ferric iron complex, the ferrioxalate ion. Oxalic acid typically occurs as the dihydrate with the formula . History The preparation of salts of oxalic acid from plants had been known since at least 1745, when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkalinity
Alkalinity (from ) is the capacity of water to resist Freshwater acidification, acidification. It should not be confused with base (chemistry), basicity, which is an absolute measurement on the pH scale. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate acid, conjugate bases. It is measured by Titration, titrating the Solution (chemistry), solution with an acid such as HCl until its pH changes abruptly, or it reaches a known endpoint where that happens. Alkalinity is expressed in units of concentration, such as meq/L (milliequivalents per liter), μeq/kg (microequivalents per kilogram), or mg/L CaCO3 (milligrams per liter of calcium carbonate). Each of these measurements corresponds to an amount of acid added as a titrant. In Fresh water, freshwater, particularly those on non-limestone terrains, alkalinities are low and involve a lot of ions. In the ocean, on the other hand, alkalinity is completely dominated by carbonate and bicarbonate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limewater
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed with water. Annually, approximately 125 million tons of calcium hydroxide are produced worldwide. Calcium hydroxide has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Solubility Calcium hydroxide is moderately soluble in water, as seen for many dihydroxides. Its solubility increases from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. Its solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the followin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonic Acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters. In biochemistry and physiology, the name "carbonic acid" is sometimes applied to aqueous solutions of carbon dioxide. These chemical species play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis. Terminology in biochemical literature In chemistry, the term "carbonic acid" strictly refers to the chemical compound with the formula . Some biochemistry literature effaces the distinction between carbonic acid and carbon dioxide dissolved in extracellular fluid. In physiology, carbon dioxide excreted by the lungs may be called ''volatile acid'' or ''respiratory acid''. Anh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bile Acid
Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Diverse bile acids are synthesized in the liver in peroxisomes. Bile acids are conjugated with taurine or glycine residues to give anions called bile salts. Primary bile acids are those synthesized by the liver. Secondary bile acids result from bacterial actions in the colon. In humans, taurocholic acid and glycocholic acid (derivatives of cholic acid) and taurochenodeoxycholic acid and glycochenodeoxycholic acid (derivatives of chenodeoxycholic acid) are the major bile salts. The salts of their 7-alpha-dehydroxylated derivatives, deoxycholic acid and lithocholic acid, are also found, with derivatives of cholic, chenodeoxycholic and deoxycholic acids accounting for over 90% of human biliary bile acids. Description Bile acids comprise about 80% of the organic compounds in bile (others are phospholipids and cholesterol). An increased secretion of bile acids produces an increase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aventurine
Aventurine is a form of quartzite, characterised by its translucency and the presence of platy mineral inclusions that give it a shimmering or glistening effect termed '' aventurescence''. Background The most common color of aventurine is green, but it can also be orange, brown, yellow, blue, or grey. Chrome-bearing fuchsite (a variety of muscovite mica) is the classic inclusion and gives a silvery green or blue sheen. Oranges and browns are attributed to hematite or goethite. Because aventurine is a rock, its physical properties vary: its specific gravity may lie between 2.64–2.69 and its hardness is somewhat lower than single-crystal quartz at around 6.5. Aventurine feldspar or sunstone can be confused with orange and red aventurine quartzite, although the former is generally of a higher transparency. Aventurine is often banded and an overabundance of fuchsite may render it opaque, in which case it may be mistaken for malachite at first glance. The name ''aventurine'' de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dmitri Mendeleev
Dmitri Ivanovich Mendeleev ( ; ) was a Russian chemist known for formulating the periodic law and creating a version of the periodic table of elements. He used the periodic law not only to correct the then-accepted properties of some known elements, such as the valence and atomic weight of uranium, but also to predict the properties of three elements that were yet to be discovered (germanium, gallium and scandium). Early life Mendeleev was born in the village of Verkhnie Aremzyani, near Tobolsk in Siberia, to Ivan Pavlovich Mendeleev (1783–1847) and Maria Dmitrievna Mendeleeva (née Kornilieva) (1793–1850).''Maria Mendeleeva (1951)''. D. I. Mendeleev's Archive: Autobiographical Writings. Collection of Documents. Volume 1 /Biographical notes about D. I. Mendeleev (written by me – D. Mendeleev), p. 13 – Leningrad: D. I. Mendeleev's Museum-Archive, 207 pages (in Russian) Ivan worked as a school principal and a teacher of fine arts, politics and philosophy at the Tambov an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics. Vertical, horizontal and diagonal trends characterize the periodic table. Metallic character increases going down a group and from right to left across a period. Nonmetallic character increases going from the bottom left of the periodic table to the top right. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869; he formulated the periodic law as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its atomic nucleus, nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements form Homonuclear molecule, molecules of atoms of said element only: e.g. atoms of hydrogen (H) form Diatomic molecule, diatomic molecules (H). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Mass
Atomic mass ( or ) is the mass of a single atom. The atomic mass mostly comes from the combined mass of the protons and neutrons in the nucleus, with minor contributions from the electrons and nuclear binding energy. The atomic mass of atoms, ions, or atomic nuclei is slightly less than the sum of the masses of their constituent protons, neutrons, and electrons, due to mass defect (explained by mass-energy equivalence: ). Atomic mass is often measured in dalton (Da) or unified atomic mass unit (u). One dalton is equal to the mass of a carbon-12 atom in its natural state, given by the atomic mass constant , where is the atomic mass of carbon-12. Thus, the numerical value of the atomic mass of a nuclide when expressed in daltons is close to its mass number. The relative isotopic mass (see section below) can be obtained by dividing the atomic mass of an isotope by the atomic mass constant , yielding a dimensionless value. Thus, the atomic mass of a carbon-12 atom is b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |