|
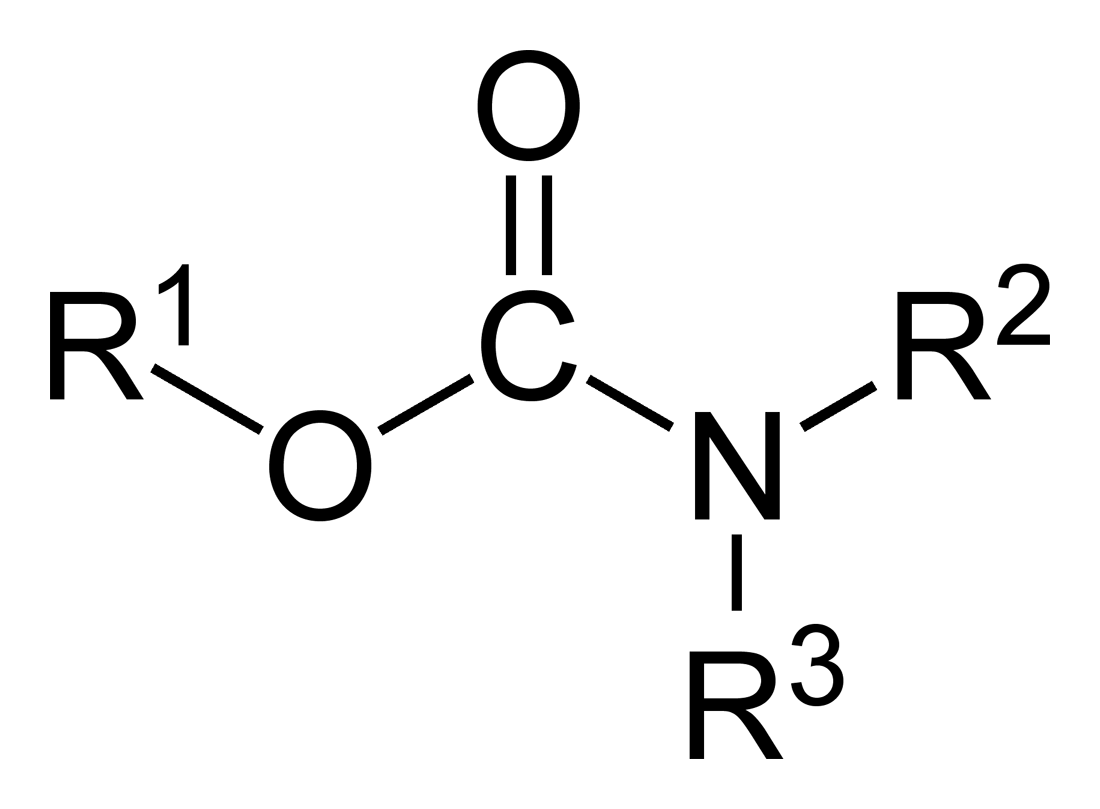
Mannich Bases
A Mannich base is a beta-amino-ketone, which is formed in the reaction of an amine, formaldehyde (or an aldehyde) and a carbon acid. The Mannich base is an endproduct in the Mannich reaction, which is nucleophilic addition reaction of a non-enolizable aldehyde and any primary or secondary amine to produce resonance stabilized imine (iminium ion or imine salt). The addition of a carbanion from a CH acidic compound (any enolizable carbonyl compound, amide, carbamate, hydantoin or urea) to the imine gives the Mannich base.Mannich base applied in this example: Reactivity With primary or secondary amines, Mannich bases react with additional aldehyde and carbon acid to larger adducts HN(CH2CH2COR)2 and N(CH2CH2COR)3. With multiple acidic hydrogen atoms on the carbon acid higher adducts are also possible. Ammonia can be split off in an elimination reaction An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general Chemical formula, formula and Chemical structure, structure , which are formally Derivative (chemistry), derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salt (chemistry), salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose repeat units are joined by carbamate like groups are an important family of plastics, the polyurethanes. See for clarification. Properties While carbamic acids are unstable, many carbamate esters and salt (chemistry), salts are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elimination Reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1cB-elimination reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism, Ei mechanism. E2 mechanism The E2 mechanism, where E2 stands for bimolecular elimination, involves a one-step mechanism in which ''carbon-hydrogen'' and ''carbon-halogen'' bonds break to form a double bond (''C=C molecular geometry, Pi bond''). The specifics of the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Open Access (publishing)
Open access (OA) is a set of principles and a range of practices through which nominally copyrightable publications are delivered to readers free of access charges or other barriers. With open access strictly defined (according to the 2001 definition), or libre open access, barriers to copying or reuse are also reduced or removed by applying an open license for copyright, which regulates post-publication uses of the work. The main focus of the open access movement has been on "peer reviewed research literature", and more specifically on academic journals. This is because: * such publications have been a subject of serials crisis, unlike newspapers, magazines and fiction writing. The main difference between these two groups is in demand elasticity: whereas an English literature curriculum can substitute '' Harry Potter and the Philosopher's Stone'' with a free-domain alternative, such as '' A Voyage to Lilliput,'' an emergency room physician treating a patient for a lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The Brazilian Chemical Society
The Journal of the Brazilian Chemical Society (print , eISSN , CODEN JOCSET) is a Brazilian scientific journal in chemistry. It was founded in 1990 and is published by the '' Brazilian Society of Chemistry'' (''Sociedade Brasileira de Química''), located at the Instituto de Química da Universidade de São Paulo. The journal is online, and the full text is freely available. According to the ''Journal Citation Reports'', the journal has a 2014 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a type of journal ranking. Journals with higher impact factor values are considered more prestigious or important within their field. The Impact Factor of a journa ... of 1.129, ranking it 100th out of 157 journals in the category "Chemistry Multidisciplinary". The ' publishes other chemistry journals with the titles '' Química Nova'' and ''Química Nova na Escola'' (QNEsc). The Journal of the Brazilian Chemical Society should not be confoun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions. Distinction is sometimes made between aldimines and ketimines, derived from aldehydes and ketones, respectively. Structure In imines the five core atoms (C2C=NX, ketimine; and C(H)C=NX, aldimine; X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually double-bonded carbon and the nitrogen atoms. The C=N distance is 1.29–1.31 Å for nonconjugated imines and 1.35 Å for conjugated imines. By contrast, C−N distances in amines and nitriles are 1.47 and 1.16 Å respectively. Rotation about the C=N bond is slow. Using NMR spectroscopy, both E–Z notation, ''E'' and ''Z'' isomers of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' are methyl), with the formula . Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, ''e.g.'', testosterone, and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered retained IUPAC names, although some introdu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene"/"alkene" and the "-ol". Many kinds of enols are known. Keto–enol tautomerism refers to a chemical equilibrium between a "keto" form (a carbonyl, named for the common ketone case) and an enol. The interconversion of the two forms involves the transfer of an alpha hydrogen atom and the reorganisation of bonding electrons. The keto and enol forms are tautomers of each other. Enolization Organic esters, ketones, and aldehydes with an α-hydrogen ( bond adjacent to the carbonyl group) often form enols. The reaction involves migration of a proton () from carbon to oxygen: : In the case of ketones, the conversion is called a keto-enol tautomerism, although this name is often more generally applied to all such tautomerizations. Usua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Addition Reaction
In organic chemistry, an addition reaction is an organic reaction in which two or more molecule A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...s combine to form a larger molecule called the '' adduct''.. An addition reaction is limited to chemical compounds that have multiple bonds. Examples include a molecule with a carbon–carbon double bond (an alkene) or a triple bond (an alkyne). Another example is a compound that has rings (which are also considered points of unsaturation). A molecule that has carbon— heteroatom double bonds, such as a carbonyl group () or imine group (), can undergo an addition reaction because its double-bond. An addition reaction is the reverse of an elimination reaction, in which one molecule divides into two or more molecules. For insta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |