|
Maltase Reaction
Maltase (, ''alpha-glucosidase'', ''glucoinvertase'', ''glucosidosucrase'', ''maltase-glucoamylase'', ''alpha-glucopyranosidase'', ''glucosidoinvertase'', ''alpha-D-glucosidase'', ''alpha-glucoside hydrolase'', ''alpha-1,4-glucosidase'', ''alpha-D-glucoside glucohydrolase'') is one type of alpha-glucosidase enzymes located in the brush border of the small intestine. This enzyme catalyzes the hydrolysis of disaccharide maltose into two simple sugars of glucose. Maltase is found in plants, bacteria, yeast, humans, and other vertebrates. It is thought to be synthesized by cells of the mucous membrane lining the intestinal wall. Digestion of starch requires six intestinal enzymes. Two of these enzymes are luminal endo-glucosidases named alpha-amylases. The other four enzymes have been identified as different maltases, exo-glucosidases bound to the luminal surface of enterocytes. Two of these maltase activities were associated with sucrase-isomaltase (maltase Ib, maltase Ia). The ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltose Structure
} Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar. History Maltose was discovered by Augustin-Pierre Dubrunfaut, although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer Cornelius O'Sullivan. Its name comes from malt, combined with the suffix '-ose' which is used in names of sugars. Structure and nomenclature Carbohydrates are generally divided into monosaccharides, oligosaccharides, and polysaccharides depending on the numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base ( adenine), the sugar ribose, and the triphosphate. Structure ATP consists of an adenine attached by the 9-nitrogen atom to the 1′ carbon atom of a sugar ( ribose), which in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alzheimer's Disease
Alzheimer's disease (AD) is a neurodegeneration, neurodegenerative disease that usually starts slowly and progressively worsens. It is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in short-term memory, remembering recent events. As the disease advances, symptoms can include primary progressive aphasia, problems with language, Orientation (mental), disorientation (including easily getting lost), mood swings, loss of motivation, self-neglect, and challenging behaviour, behavioral issues. As a person's condition declines, they often withdraw from family and society. Gradually, bodily functions are lost, ultimately leading to death. Although the speed of progression can vary, the typical life expectancy following diagnosis is three to nine years. The cause of Alzheimer's disease is poorly understood. There are many environmental and genetic risk factors associated with its development. The strongest genetic risk factor is from an alle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AMY1A
Alpha-amylase 1 is an enzyme that in humans is encoded by the ''AMY1A'' gene. This gene is found in many organisms. Amylases are secreted proteins that hydrolyze 1,4-alpha-glucoside bonds in oligosaccharides and polysaccharides, and thus catalyze the first step in digestion of dietary starch and glycogen. The human genome has a cluster of several amylase genes that are expressed at high levels in either salivary gland or pancreas. This gene encodes an amylase isoenzyme produced by the salivary gland. Alternative splicing results in multiple transcript variants encoding the same protein. See also * AMY2A Pancreatic alpha-amylase is an enzyme that in humans is encoded by the ''AMY2A'' gene. Amylases are secreted proteins that hydrolyze 1,4-alpha-glucoside bonds in oligosaccharides and polysaccharides, and thus catalyze the first step in digestion ... * References External links * * Further reading * * * * * * * * * * * * * * * * * * * * {{enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sucrase-isomaltase
Oligo-1,6-glucosidase (EC 3.2.1.10, sucrase-isomaltase, SI; systematic name oligosaccharide 6-α-glucohydrolase) is a glucosidase enzyme located on the brush border of the small intestine, which catalyses the following reaction: :Hydrolysis of (1→6)-α-D-glucosidic linkages in some oligosaccharides produced from starch and glycogen by (α-amylase), and in isomaltose It is a dual-function enzyme with two GH31 domains, one serving as the isomaltase, the other as a sucrose alpha-glucosidase. It has preferential expression in the apical membranes of enterocytes. The enzyme’s purpose is to digest dietary carbohydrates such as starch, sucrose and isomaltose. By further processing the broken-down products, energy in the form of ATP can be generated.Berg, J. M. et al. ''Biochemistry'', 7th Ed. W.H. Freeman and Company: New York, 2012. Structure Sucrase-isomaltase consists of two enzymatic subunits: sucrase and isomaltase. The subunits originate from a polypeptide precu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltase-glucoamylase
Maltase-glucoamylase, intestinal is an enzyme that in humans is encoded by the ''MGAM'' gene. Maltase-glucoamylase is an alpha-glucosidase digestive enzyme. It consists of two subunits with differing substrate specificity. Recombinant enzyme studies have shown that its N-terminal catalytic domain has highest activity against maltose, while the C-terminal domain has a broader substrate specificity and activity against glucose oligomers. In the small intestine, this enzyme works in synergy with sucrase-isomaltase and alpha-amylase to digest the full range of dietary starches. Gene The MGAM gene –– which is located on chromosome 7q34 –– codes for the protein Maltase-Glucoamylase. An alternative name for Maltase-Glucoamylase is glucan 1,4-alpha-glycosidase. Tissue distribution Maltase-glucoamylase is a membrane-bound enzyme located in the intestinal walls. This lining of the intestine forms brush border in which food has to pass in order for the intestines to absor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body. Glycogen functions as one of two forms of energy reserves, glycogen being for short-term and the other form being triglyceride stores in adipose tissue (i.e., body fat) for long-term storage. In humans, glycogen is made and stored primarily in the cells of the liver and skeletal muscle. In the liver, glycogen can make up 5–6% of the organ's fresh weight, and the liver of an adult, weighing 1.5 kg, can store roughly 100–120 grams of glycogen. In skeletal muscle, glycogen is found in a low concentration (1–2% of the muscle mass) and the skeletal muscle of an adult weighing 70 kg stores roughly 400 grams of glycogen. The amount of glycogen stored in the body—particularly within the muscles and liver—mostly depends on physical training, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Alpha-glucosidase
Acid alpha-glucosidase, also called α-1,4-glucosidase and acid maltase, is an enzyme () that helps to break down glycogen in the lysosome. It is functionally similar to glycogen debranching enzyme, but is on a different chromosome, processed differently by the cell and is located in the lysosome rather than the cytosol. In humans, it is encoded by the ''GAA'' gene. Errors in this gene cause glycogen storage disease type II (Pompe disease). Function This gene encodes lysosomal alpha-glucosidase, which is essential for the degradation of glycogen to glucose in lysosome A lysosome () is a membrane-bound organelle found in many animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules. A lysosome has a specific composition, of both its membrane ...s. Different forms of acid alpha-glucosidase are obtained by proteolytic processing. Defects in this gene are the cause of glycogen storage disease II, also known a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellular Respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions, which break large molecules into smaller ones, releasing energy. Respiration is one of the key ways a cell releases chemical energy to fuel cellular activity. The overall reaction occurs in a series of biochemical steps, some of which are redox reactions. Although cellular respiration is technically a combustion reaction, it is an unusual one because of the slow, controlled release of energy from the series of reactions. Nutrients that are commonly used by animal and pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside Hydrolase Family 13
In molecular biology, glycoside hydrolase family 13 is a family of glycoside hydrolases. Glycoside hydrolases are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycoside hydrolases, based on sequence similarity, has led to the definition of >100 different families. This classification is available on the CAZy web site, and also discussed at CAZypedia, an online encyclopedia of carbohydrate active enzymes. Enzymes containing this domain belong to family 13CAZY GH_13 of the glycosyl hydrolases. The maltogenic alpha-amylase is an enzyme which catalyses hydrolysis of (1-4)-alpha-D-glucosidic linkages in polysaccharides so as to remove successive alpha-maltose residues from the non-reducing ends of the chains in the conversion of starch to maltose. Other enzymes in this family include neopullulanase, which hydrolyses pullulan to panose, and cy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
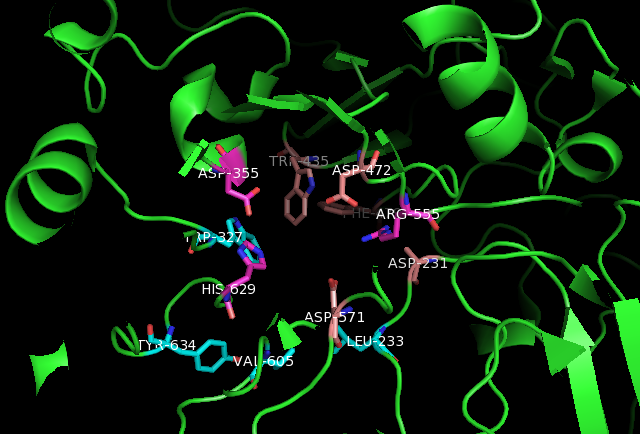
Maltase-Glucoamylase Ligand Interactions
Maltase-glucoamylase, intestinal is an enzyme that in humans is encoded by the ''MGAM'' gene. Maltase-glucoamylase is an alpha-glucosidase digestive enzyme. It consists of two subunits with differing substrate specificity. Recombinant enzyme studies have shown that its N-terminal catalytic domain has highest activity against maltose, while the C-terminal domain has a broader substrate specificity and activity against glucose oligomers. In the small intestine, this enzyme works in synergy with sucrase-isomaltase and alpha-amylase to digest the full range of starch, dietary starches. Gene The MGAM gene –– which is located on chromosome 7q34 –– codes for the protein Maltase-Glucoamylase. An alternative name for Maltase-Glucoamylase is glucan 1,4-alpha-glycosidase. Tissue distribution Maltase-glucoamylase is a membrane-bound enzyme located in the intestinal walls. This lining of the intestine forms brush border in which food has to pass in order for the intestines to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vampire Bats
Vampire bats, species of the subfamily Desmodontinae, are leaf-nosed bats found in Central and South America. Their food source is blood of other animals, a dietary trait called hematophagy. Three extant bat species feed solely on blood: the common vampire bat (''Desmodus rotundus''), the hairy-legged vampire bat (''Diphylla ecaudata''), and the white-winged vampire bat (''Diaemus youngi''). All three species are native to the Americas, ranging from Mexico to Brazil, Chile, Uruguay and Argentina. Taxonomy Due to differences among the three species, each has been placed within a different genus, each consisting of one extant species. In the older literature, these three genera were placed within a family of their own, Desmodontidae, but taxonomists have now grouped them as a subfamily, Desmodontinae, in the New World leaf-nosed bat family, Phyllostomidae. The three known species of vampire bats all seem more similar to one another than to any other species. That suggests tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |