|
Malonyl CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid. Biosynthesis Malonyl-CoA cannot cross membranes and there is no known malonyl-CoA import mechanism. The biosynthesis therefore takes place locally: * cytosol: Malonyl-CoA is formed by carboxylating acetyl-CoA using the highly regulated enzyme acetyl-CoA carboxylase 1 (ACC1). One molecule of acetyl-CoA joins with a molecule of bicarbonate, requiring energy rendered from ATP. * Mitochondrial outer membrane: Malonyl-CoA is formed by carboxylating acetyl-CoA using the highly regulated enzyme acetyl-CoA carboxylase 2 (ACC2). The reaction is the same as with ACC1. * mitochondrial matrix: Malonyl-CoA is formed in coordinated fashion by mtACC1, a mitochondrial isoform of ACC1, and acyl-CoA synthetase family member 3 (ACSF3), a mitochondrial malonyl-CoA synthetase. MtACC1, like cytosolic ACC1 catalyses the carboxylation of acetyl-CoA, while ACSF3 catalyses the thioesterification of malonate to coenzyme A. The latter serves fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the Fatty acid metabolism#Synthesis, synthesis and Fatty acid metabolism#.CE.B2-Oxidation, oxidation of fatty acids, and the oxidation of pyruvic acid, pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a Substrate (chemistry), substrate, and around 4% of cellular enzymes use it (or a thioester) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenic acid, pantothenate (vitamin B5), and adenosine triphosphate (ATP). In acetyl-CoA, its acetyl form, coenzyme A is a highly versatile molecule, serving metabolic functions in both the Anabolism, anabolic and Catabolism, catabolic pathways. Acetyl-CoA is utilised in the post-translational regulation and allosteric regulation of pyruvate dehydrogenase and carboxylase to maintain and support the partition of Pyruvic acid, pyruvate synthesis and degradation. Discovery of structure Coenzyme A was ident ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
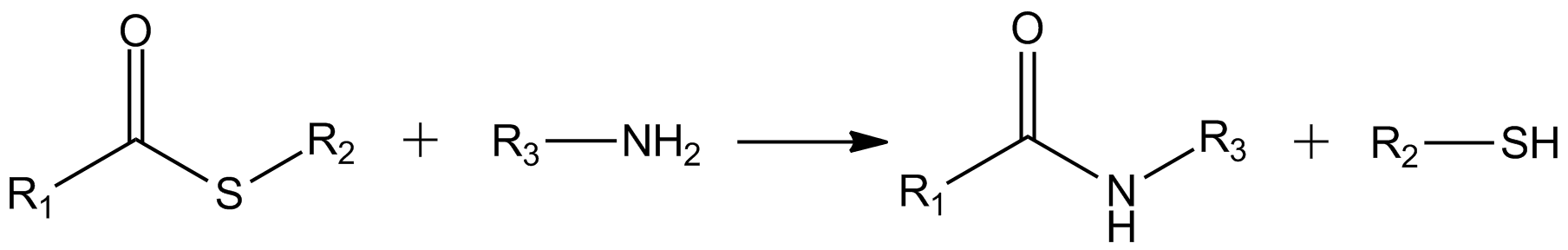
Thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid () with a thiol (). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. The R and R' represent organyl groups, or H in the case of R. Synthesis One route to thioesters involves the reaction of an acid chloride with an alkali metal salt of a thiol: : Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation of potassium thioacetate: : Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty Acids
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids (up to 70% by weight) in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells. History The concept of fatty acid (''acide gras'') was introduced in 1813 by Michel Eugène Chevreul, though he initially used some variant terms: ''graisse acide'' and ''acide huileux'' ("acid fat" and "oily acid"). Types of fatty acids Fatty acids are classified in many ways: by length, by saturation vs unsat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-oxidation
In biochemistry and metabolism, beta oxidation (also β-oxidation) is the Catabolism, catabolic process by which fatty acid molecules are broken down in the cytosol in prokaryotes and in the mitochondria in eukaryotes to generate acetyl-CoA. Acetyl-CoA enters the citric acid cycle, generating NADH and FADH2, FADH2, which are electron carriers used in the electron transport chain. It is named as such because the Alpha and beta carbon, beta carbon of the fatty acid chain undergoes oxidation and is converted to a carbonyl group to start the cycle all over again. Beta-oxidation is primarily facilitated by the mitochondrial trifunctional protein, an enzyme complex associated with the inner mitochondrial membrane, although Very long chain fatty acid, very long chain fatty acids are oxidized in Peroxisome, peroxisomes. The overall reaction for one cycle of beta oxidation is: :C''n''-acyl-CoA + FAD + NAD''+'' + H''2''O + CoA → C''n''-2-acyl-CoA + FADH''2'' + NADH + H''+'' + acetyl-CoA A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyl Carrier Protein
The acyl carrier protein (ACP) is a cofactor of both fatty acid and polyketide biosynthesis machinery. It is one of the most abundant proteins in cells of ''E. coli.'' In both cases, the growing chain is bound to the ACP via a thioester derived from the distal thiol of a 4'-phosphopantetheine moiety. Structure The ACPs are small negatively charged α-helical bundle proteins with a high degree of structural and amino acid similarity. The structures of a number of acyl carrier proteins have been solved using various NMR and crystallography techniques. The ACPs are related in structure and mechanism to the peptidyl carrier proteins (PCP) from nonribosomal peptide synthases. Biosynthesis Subsequent to the expression of the inactive ''apo'' ACP, the 4'-phosphopantetheine moiety is attached to a serine residue. This coupling is mediated by acyl carrier protein synthase (ACPS), a 4'-phosphopantetheinyl transferase. 4'-Phosphopantetheine is a prosthetic group of several acyl carrier pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic, cabbage or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the smell of natural gas is due to the smell of the thiol used as the odorant. Nomenclature Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyl Carrier Protein Transacylase
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group () or hydrogen in the case of formyl group (). In organic chemistry, the acyl group (IUPAC name alkanoyl if the organyl group is alkyl) is usually derived from a carboxylic acid, in which case it has the formula , where R represents an organyl group or hydrogen. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids and phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond. Reactivity trends There are five main types of acyl derivatives. Acid halides are the most reactive towards nucleophiles, followed by anhydrides, esters, and amides. Carboxylate ions are essentia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyketide
In organic chemistry, polyketides are a class of natural products derived from a Precursor (chemistry), precursor molecule consisting of a Polymer backbone, chain of alternating ketone (, or Carbonyl reduction, its reduced forms) and Methylene group, methylene () groups: . First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity. History Naturally produced polyketides by various plants and organisms have been used by humans since before studies on them began in the 19th and 20th century ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty Acid Biosynthesis
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes. Two '' de novo'' fatty acid syntheses can be distinguished: cytosolic fatty acid synthesis (FAS/FASI) and mitochondrial fatty acid synthesis (mtFAS/mtFASII). Most of the acetyl-CoA which is converted into fatty acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway also provides the glycerol with which three fatty acids can combine (by means of ester bonds) to form triglycerides (also known as "triacylglycerols" – to distinguish them from fatty "acids" – or simply as "fat"), the final product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol group is phosphorylated with a group such as phosphatidylcholine, a phospholipid is formed. Phospholipids form the bulk of the lipid bilayers that make up cell membranes and surrounds the organelles within the cells (such as the ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
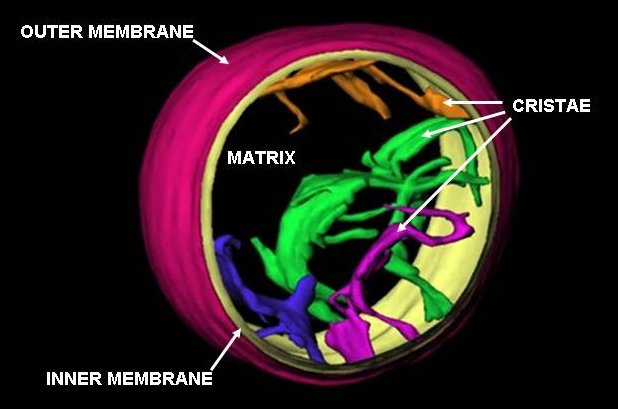
Mitochondrion
A mitochondrion () is an organelle found in the cell (biology), cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'', meaning a thread-like granule, was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase popularized by Philip Siekevitz in a 1957 ''Scientific American'' article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). The multicellular animal ''Henneguya zschokkei, Henneguya salminicola'' is known to have retained mitochondrion-related organelles despite a complete loss of their mitochondrial genome. A large number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinate Dehydrogenase
Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates in both the citric acid cycle and oxidative phosphorylation. Histochemical analysis showing high succinate dehydrogenase in muscle demonstrates high mitochondrial content and high oxidative potential. In step 6 of the citric acid cycle, SQR catalyzes the oxidation of succinate to fumarate with the reduction of ubiquinone to ubiquinol. This occurs in the inner mitochondrial membrane by coupling the two reactions together. Structure Subunits Mitochondrial and many bacterial SQRs are composed of four structurally different subunits: two hydrophilic and two hydrophobic. The first two subunits, a flavoprotein (SDHA) and an iron-sulfur protein (SDHB), form a hydrophilic head where enzymatic activity of the complex takes place. SD ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |