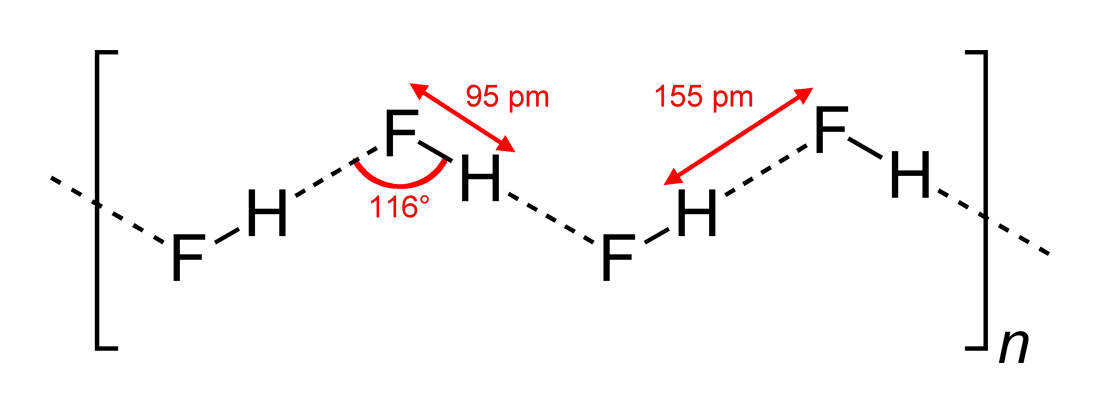
|
Lutetium Trifluoride
Lutetium(III) fluoride is an inorganic compound with a chemical formula LuF3. Production Lutetium(III) fluoride can be produced by reacting lutetium oxide with hydrogen fluoride, or reacting lutetium chloride and hydrofluoric acid: : : It can also be produced by reacting lutetium sulfide and hydrofluoric acid: : (x = 0.9) : Lutetium oxide and nitrogen trifluoride Nitrogen trifluoride is the inorganic compound with the formula (). It is a colorless, non-flammable, toxic gas with a slightly musty odor. In contrast with ammonia, it is nonbasic. It finds increasing use within the manufacturing of flat-panel ... react at 240 °C to produce LuOF. A second step happens below 460 °C to produce LuF3. References Lutetium compounds Fluorides Lanthanide halides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lutetium(III) Chloride
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic white monoclinic crystals and also a hygroscopic hexahydrate LuCl3·6H2O. Anhydrous lutetium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral lutetium ions. Lutetium-177, a radioisotope that can be derived from lutetium(III) chloride, is used in targeted cancer therapies. When lutetium-177 is attached to molecules that specifically target cancer cells, it can deliver localized radiation to destroy those cells while sparing surrounding healthy tissue. This makes lutetium-177-based treatments especially valuable for cancers that are difficult to treat with traditional methods, such as neuroendocrine tumors and prostate cancer. Additionally, lutetium(III) chloride is used in scintillators, materials that emit light when exposed to radiation. These scintillators are crucial in detectors for gamma rays and other high-en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lutetium(III) Bromide
Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted among the rare earth elements; it can also be classified as the first element of the 6th-period transition metals. Lutetium was independently discovered in 1907 by French scientist Georges Urbain, Austrian mineralogist Baron Carl Auer von Welsbach, and American chemist Charles James. All of these researchers found lutetium as an impurity in ytterbium. The dispute on the priority of the discovery occurred shortly after, with Urbain and Welsbach accusing each other of publishing results influenced by the published research of the other; the naming honor went to Urbain, as he had published his results earlier. He chose the name ''lutecium'' for the new element, but in 1949 the spelling was changed to ''lutetium''. In 1909, the priority was fina ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scandium(III) Fluoride
Scandium(III) fluoride, ScF3, is an ionic compound. This salt is slightly soluble in water but dissolves in the presence of excess fluoride to form the ScF63− anion. Production ScF3 can be produced by reacting scandium and fluorine.S.A.Cotton, Scandium, Yttrium and the Lanthanides: Inorganic and Coordination Chemistry, Encyclopedia of Inorganic Chemistry, 1994, John Wiley & Sons, . It is also formed during the extraction from the ore thortveitite by the reaction of Sc2O3 with ammonium bifluoride at high temperature:Pradyot Patnaik, 2003, ''Handbook of Inorganic Chemicals'', McGraw-Hill Professional, . : Sc2O3 + 6 NH4HF2 → 2 ScF3 + 6 NH4F + 3 H2O The resulting mixture contains a number of metal fluorides and this is reduced by reaction with calcium metal at high temperature. Further purification steps are required to produce usable metallic scandium. Properties Scandium trifluoride exhibits the unusual property of negative thermal expansion, meaning it shrinks when heated. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium(III) Fluoride
Yttrium(III) fluoride is an inorganic chemical compound with the chemical formula Y F3. It is not known naturally in "pure" form. The fluoride minerals containing essential yttrium include tveitite-(Y) (Y,Na)6Ca6Ca6F42 and gagarinite-(Y) NaCaY(F,Cl)6. Sometimes mineral fluorite contains admixtures of yttrium. Synthesis YF3 can be produced by reacting fluorine with yttria or yttrium hydroxide with hydrofluoric acid. :Y(OH)3 + 3HF → YF3 + 3H2O Properties Yttrium(III) fluoride has a refractive index of 1.51 at 500 nm and is transparent in the range from 193 nm to 14,000 nm (i.e. from the UV to IR range). Pure yttrium can be obtained from yttrium(III) fluoride by reduction with calcium. Yttrium(III) fluoride crystallizes in the orthorhombic crystal system, with space group Pnma (space group no. 62), with the lattice parameters a = 6.3537 Å, b = 6.8545 Å, c = 4.3953 Å. Yttrium is nine times coordinated by fluorine atoms. Occurrence and uses It occurs as the mineral waim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep Mantle (geology), mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salt (chemistry), salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within life, living things. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lutetium Oxide
Lutetium(III) oxide, a white solid, is a cubic compound of lutetium sometimes used in the preparation of specialty glasses. It is also called lutecia. It is a lanthanide oxide, also known as a rare earth.Lutetium Oxide. 1997-2007. Metall Rare Earth Limited. http://www.metall.com.cn/luo.htm History In 1879, Swiss chemist Jean Charles Galissard de Marignac (1817–1894) claimed to have discovered ytterbium, but he had found a mixture of elements. In 1907, French chemist Georges Urbain (1872–1938) reported that ytterbium was a mixture of two new elements and was not a single element. Two other chemists, Carl Auer von Welsbach (1858–1929) and Charles James (1880–1926) also extracted lutetium(III) oxide around the same time. All three scientists successfully separated Marignac's ytterbia into oxides of two elements which were eventually named ytterbium and lutetium Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lutetium Chloride
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic white monoclinic crystals and also a hygroscopic hexahydrate LuCl3·6H2O. Anhydrous lutetium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral lutetium ions. Lutetium-177, a radioisotope that can be derived from lutetium(III) chloride, is used in targeted cancer therapies. When lutetium-177 is attached to molecules that specifically target cancer cells, it can deliver localized radiation to destroy those cells while sparing surrounding healthy tissue. This makes lutetium-177-based treatments especially valuable for cancers that are difficult to treat with traditional methods, such as neuroendocrine tumors and prostate cancer. Additionally, lutetium(III) chloride is used in scintillators, materials that emit light when exposed to radiation. These scintillators are crucial in detectors for gamma rays and other high-en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling point near room temperature. It is used to make most organofluorine compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lutetium Sulfide
Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted among the rare earth elements; it can also be classified as the first element of the 6th-period transition metals. Lutetium was independently discovered in 1907 by French scientist Georges Urbain, Austrian mineralogist Baron Carl Auer von Welsbach, and American chemist Charles James. All of these researchers found lutetium as an impurity in ytterbium. The dispute on the priority of the discovery occurred shortly after, with Urbain and Welsbach accusing each other of publishing results influenced by the published research of the other; the naming honor went to Urbain, as he had published his results earlier. He chose the name ''lutecium'' for the new element, but in 1949 the spelling was changed to ''lutetium''. In 1909, the priority was fina ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
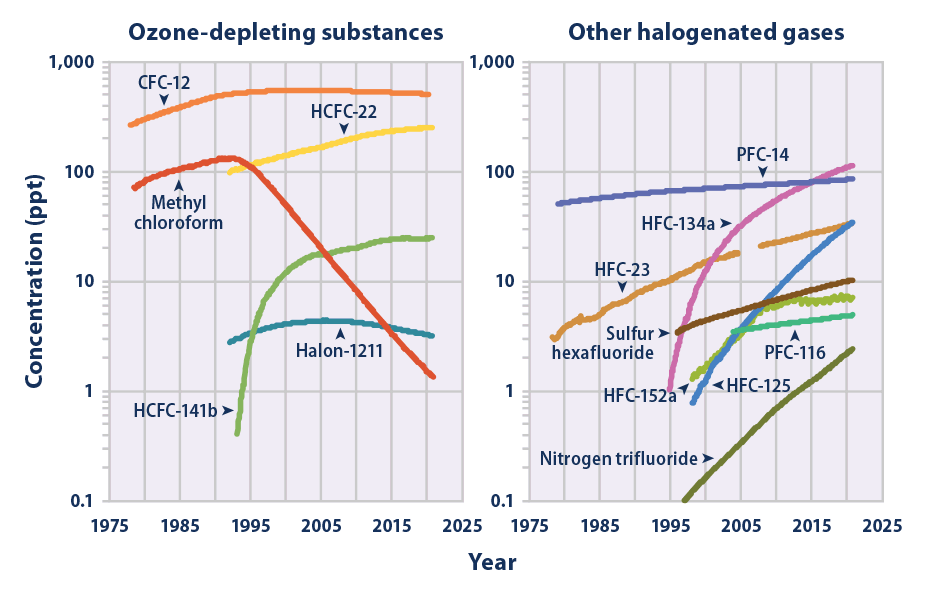
Nitrogen Trifluoride
Nitrogen trifluoride is the inorganic compound with the formula (). It is a colorless, non-flammable, toxic gas with a slightly musty odor. In contrast with ammonia, it is nonbasic. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. is a greenhouse gas, with a global warming potential (GWP) 17,200 times greater than that of when compared over a 100-year period. Synthesis and reactivity Nitrogen trifluoride can be prepared from the elements in the presence of an electric discharge. In 1903, Otto Ruff prepared nitrogen trifluoride by the electrolysis of a molten mixture of ammonium fluoride and hydrogen fluoride. It is far less reactive than the other nitrogen trihalides nitrogen trichloride, nitrogen tribromide, and nitrogen triiodide, all of which are explosive. Alone among the nitrogen trihalides it has a negative enthalpy of formation. It is prepared in modern times both by direct reaction of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |