|
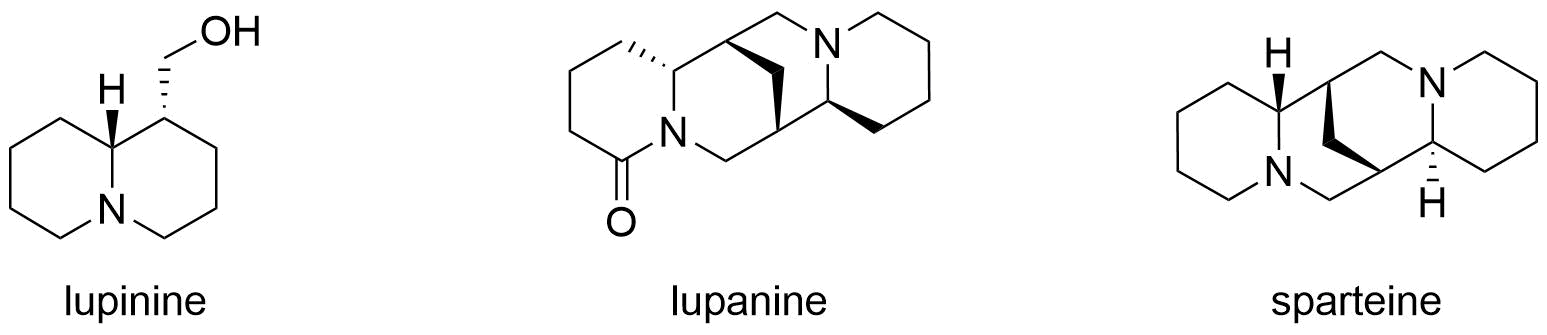
Lupinine
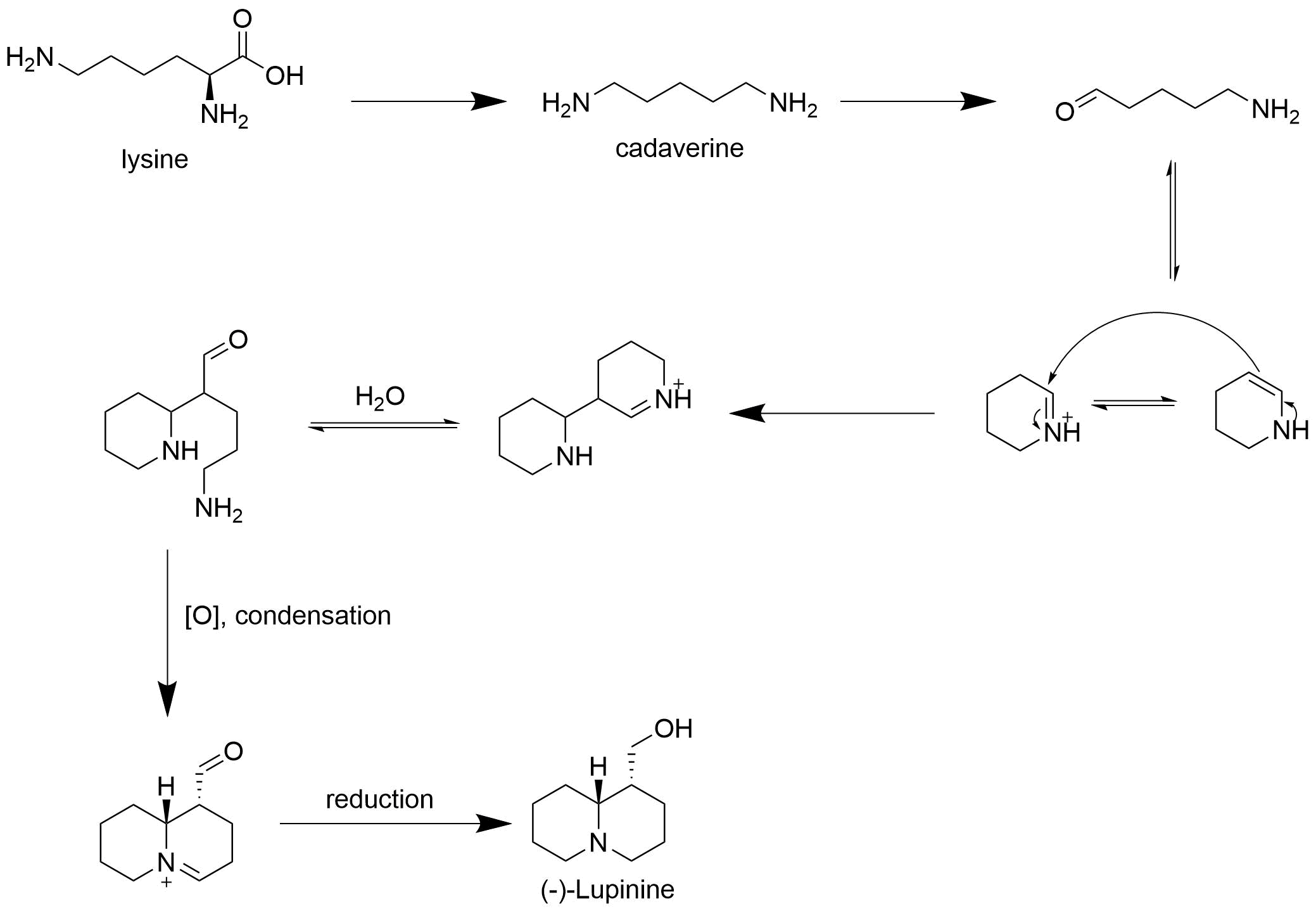
Lupinine is a quinolizidine alkaloid present in the genus ''Lupinus'' (colloquially referred to as lupins) of the flowering plant family Fabaceae. The scientific literature contains many reports on the isolation and synthesis of this compound as well as a vast number of studies on its biosynthesis from its natural precursor, lysine. Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor and that lupinine has an inhibitory effect on acetylcholine receptors. The characteristically bitter taste of lupin beans, which come from the seeds of ''Lupinus'' plants, is attributable to the quinolizidine alkaloids which they contain, rendering them unsuitable for human and animal consumption unless handled properly. However, because lupin beans have potential nutritional value due to their high protein content, efforts have been made to reduce their alkaloid content through the development of "sweet" varieties of ''Lupinus''. Toxicity Lupinine is a he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lupinine Graphic 1
Lupinine is a Quinolizidine alkaloids, quinolizidine alkaloid present in the genus ''Lupinus'' (colloquially referred to as lupins) of the flowering plant family Fabaceae. The scientific literature contains many reports on the isolation and synthesis of this compound as well as a vast number of studies on its biosynthesis from its natural precursor, lysine. Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor and that lupinine has an inhibitory effect on acetylcholine receptors. The characteristically bitter taste of lupin beans, which come from the seeds of ''Lupinus'' plants, is attributable to the quinolizidine alkaloids which they contain, rendering them unsuitable for human and animal consumption unless handled properly. However, because lupin beans have potential nutritional value due to their high protein content, efforts have been made to reduce their alkaloid content through the development of "sweet" varieties of ''Lupinus''. To ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lupinine Graphic 2
Lupinine is a quinolizidine alkaloid present in the genus ''Lupinus'' (colloquially referred to as lupins) of the flowering plant family Fabaceae. The scientific literature contains many reports on the isolation and synthesis of this compound as well as a vast number of studies on its biosynthesis from its natural precursor, lysine. Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor and that lupinine has an inhibitory effect on acetylcholine receptors. The characteristically bitter taste of lupin beans, which come from the seeds of ''Lupinus'' plants, is attributable to the quinolizidine alkaloids which they contain, rendering them unsuitable for human and animal consumption unless handled properly. However, because lupin beans have potential nutritional value due to their high protein content, efforts have been made to reduce their alkaloid content through the development of "sweet" varieties of ''Lupinus''. Toxicity Lupinine is a he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinolizidine Alkaloids
Quinolizidine alkaloids are natural products that have a quinolizidine structure; this includes the lupine alkaloids. Occurrence Quinolizidine alkaloids can be found in the plant family of legumes, especially in papilionaceous plants. While the lupine alkaloids (following their name) can be found in lupines, tinctorin, for example, was isolated from the dyer's broom. Examples More than 200 quinolizidine alkaloids are known which can be classified into 6 structural types: * the lupinine type with 34 known structures, including lupinine and its derivatives * the camoensine type with 6 known structures, including camoensin * the spartein type with 66 structures, including sparteine, lupanine, angustifoline * the α-pyridone type with 25 structures, including anagyrine and cytisine * the matrine type with 31 structures, including matrine * and the ormosanin type with 19 structures, including ormosanine. (–)-Lupinine Structural Formula V2.svg, Lupinine, (–)-lupinine (6R,7 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lupinus
''Lupinus'', commonly known as lupin, lupine, or regionally bluebonnet, is a genus of plants in the legume family Fabaceae. The genus includes over 199 species, with centre of diversity, centres of diversity in North America, North and South America. Smaller centres occur in North Africa and the Mediterranean Basin, Mediterranean. They are widely cultivated, both as a food source and as ornamental plants, but are invasive to some areas. Description The species are mostly herbaceous perennial plants tall, but some are annual plants and a few are bush lupin, shrubs up to tall. An exception is the ''chamis de monte'' (''Lupinus jaimehintonianus'') of Oaxaca in Mexico, which is a tree up to tall. Lupins have soft green to grey-green leaves which may be coated in silvery hairs, often densely so. The leaf blades are usually palmately divided into five to 28 leaflets, or reduced to a single leaflet in a few species of the southeastern United States and eastern South America. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lupins
''Lupinus'', commonly known as lupin, lupine, or regionally bluebonnet, is a genus of plants in the legume family Fabaceae. The genus includes over 199 species, with centres of diversity in North and South America. Smaller centres occur in North Africa and the Mediterranean. They are widely cultivated, both as a food source and as ornamental plants, but are invasive to some areas. Description The species are mostly herbaceous perennial plants tall, but some are annual plants and a few are shrubs up to tall. An exception is the ''chamis de monte'' (''Lupinus jaimehintonianus'') of Oaxaca in Mexico, which is a tree up to tall. Lupins have soft green to grey-green leaves which may be coated in silvery hairs, often densely so. The leaf blades are usually palmately divided into five to 28 leaflets, or reduced to a single leaflet in a few species of the southeastern United States and eastern South America. The flowers are produced in dense or open whorls on an erect spike, ea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthesis
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-Catalysis, catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthesis) serve as enzyme substrate (chemistry), substrates, with conversion by the living organism either into simpler or more complex Product (chemistry), products. Examples of biosynthetic pathways include those for the production of amino acids, lipid membrane components, and nucleotides, but also for the production of all classes of biological macromolecules, and of acetyl-coenzyme A, adenosine triphosphate, nicotinamide adenine dinucleotide and other key intermediate and transactional molecules needed for metabolism. Thus, in biosynthesis, any of an array of Chemical compound, compounds, from simple to complex, are converted into other compounds, and so it includes both the catabolism and anabolism (building up and breaking down) of comple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endogeny (biology)
Endogeny, in biology, refers to the property of originating or developing from within an organism, Tissue (biology), tissue, or Cell (biology), cell. For example, ''endogenous substances'', and ''endogenous processes'' are those that originate within a living system (e.g. an organism or a Cell (biology), cell). For instance, estradiol is an endogenous estrogen hormone produced within the body, whereas ethinylestradiol is an exogenous synthetic estrogen, commonly used in birth control pills. In contrast, ''Exogeny#Biology, exogenous substances'' and ''exogenous'' ''processes'' are those that originate from outside of an organism. References External links *{{Wiktionary-inline, endogeny Biology ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids. Alkaloids are produced by a large variety of organisms including bacteria, fungus, fungi, Medicinal plant, plants, and animals. They can be purified from crude extracts of these organisms by acid-base extraction, or solvent extractions followed by silica-gel column chromatography. Alkaloids have a wide range of pharmacology, pharmacological activities including antimalarial medication, antimalarial (e.g. quinine), asthma, antiasthma (e.g. ephedrine), chemotherapy, anticancer (e.g. omacetaxine mepesuccinate, homoharringtonine), cholinomimetic (e.g. galantamine), vasodilation, vasodilatory (e.g. vincamine), Antiarrhythmic agent, antiarrhythmic (e.g. quinidine), analgesic (e.g. morphine), antibacterial (e.g. chelerythrine), and anti-diabetic, antihyperglycemic activities (e.g. berb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinolizidine
Quinolizidine (norlupinane, octahydro-2''H''-quinolizine) is a nitrogen-containing heterocyclic compound. Some alkaloids (e.g. cytisine and sparteine) are derivatives of quinolizidine. Quinolizidine alkaloids Quinolizidine alkaloids, such as nupharine and related chemicals, can be found in '' Nymphaea lotus'' and other species in the family Nymphaeaceae. Quinolizidine alkaloids are also found in genistoid legumes Legumes are plants in the pea family Fabaceae (or Leguminosae), or the fruit or seeds of such plants. When used as a dry grain for human consumption, the seeds are also called pulses. Legumes are grown agriculturally, primarily for human consu .... External links * * Synthesis: {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves ." '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, beta b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a Receptor (biochemistry), receptor to produce a biological response. Receptors are Cell (biology), cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an Receptor antagonist, antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology The word originates from the Ancient Greek, Greek word (''agōnistēs''), "contestant; champion; rival" < (''agōn''), "contest, combat; exertion, struggle" < (''agō''), "I lead, lead towards, conduct; drive." Types of agonists Receptor (biochemistry), Receptors can be activated by either endogenous agonists (such as hormones and neurotransmitters) or exogenous agonists (such as medication, drugs), resulting in a biological response. A physiological agonism an ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |