|
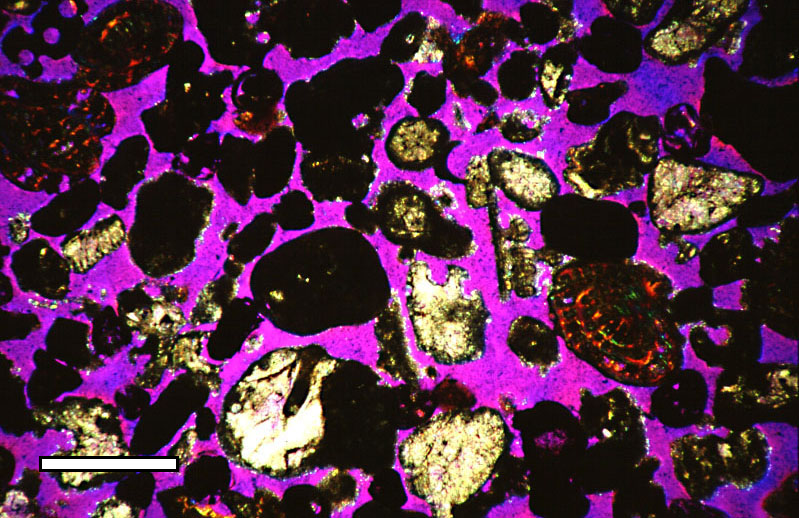
Lithify
Diagenesis () is the process that describes physical and chemical changes in sediments first caused by water-rock interactions, microbial activity, and compaction after their deposition. Increased pressure and temperature only start to play a role as sediments become buried much deeper in the Earth's crust. In the early stages, the transformation of poorly consolidated sediments into sedimentary rock (lithification) is simply accompanied by a reduction in porosity and water expulsion (clay sediments), while their main mineralogical assemblages remain unaltered. As the rock is carried deeper by further deposition above, its organic content is progressively transformed into kerogens and bitumens. The process of diagenesis excludes surface alteration (weathering) and deep metamorphism. There is no sharp boundary between diagenesis and metamorphism, but the latter occurs at higher temperatures and pressures. Hydrothermal solutions, meteoric groundwater, rock porosity, permeabili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyritized Lloydolithus Lloydi
Permineralization is a process of fossilization of bones and tissues in which mineral deposits form internal casts of organisms. Carried by water, these minerals fill the spaces within organic tissue. Because of the nature of the casts, permineralization is particularly useful in studies of the internal structures of organisms, usually of plants. Process Permineralization, a type of fossilization, involves deposits of minerals within the cells of organisms. Water from the ground, lakes, or oceans seeps into the pores of organic tissue and forms a crystal cast with deposited minerals. Crystals begin to form in the porous cell walls. This process continues on the inner surface of the walls until the central cavity of the cell, the lumen, is completely filled. The cell walls themselves remain intact surrounding the crystals. Silicification In silicification, the weathering of rocks releases silicate minerals and the silica makes its way into a body of still water. Eventually, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Archaeometry (journal)
''Archaeometry'' is a peer-reviewed academic journal covering archaeological science, particularly absolute dating methods, artefact studies, quantitative archaeology, remote sensing, conservation science, and environmental archaeology. It is published bimonthly by Wiley-Blackwell, on behalf of the Research Laboratory for Archaeology and the History of Art at the University of Oxford, in association with the ''Gesellschaft für Naturwissenschaftliche Archäologie Archäometrie'' and the Society for Archaeological Sciences. Its current editors are A. Mark Pollard, Ina Reiche, Brandi MacDonald, Gilberto Artioli, and Catherine Batt. The journal was founded as the ''Bulletin of the Research Laboratory for Archaeology and the History of Art'' in 1958. It has been published by Wiley-Blackwell since 2001. Research papers published in ''Archaeometry'' are typically "technical expositions of physical and chemical methods applicable to dating and materials identification in archaeolog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dolomitization
Dolomitization is a geological process by which the carbonate mineral dolomite is formed when magnesium ions replace calcium ions in another carbonate mineral, calcite. It is common for this mineral alteration into dolomite to take place due to evaporation of water in the sabkha area. Dolomitization involves substantial amount of recrystallization. This process is described by the stoichiometric equation: :2 CaCO3(calcite) + Mg2+ ↔ CaMg(CO3)2(dolomite) + Ca2+ Dolomitization depends on specific conditions which include low Ca:Mg ratio in solution, reactant surface area, the mineralogy of the reactant, high temperatures which represents the thermodynamic stability of the system, and the presence of kinetic inhibitors such as sulfate. If the kinetic inhibitors and high temperatures are compatible, then dolomitization can take place in saline environments above thermodynamic and kinetic saturation with respect to dolomite. This type of environment includes, freshwater and seawat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvation
Solvation (or dissolution) describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the solute, including solubility, reactivity, and color, as well as influencing the properties of the solvent such as its viscosity and density. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. The surrounded solute particles then move away from the solid solute and out into the solution. Ions are surrounded by a concentric shell of solvent. Solvation is the process of reorganizing solvent and solute molecules into solvation complexes and involves bond formation, hydrogen bonding, and van der Waals forces. Solvation of a solute by water is called hydration. Solubility of soli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porosity
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure the "accessible void", the total amount of void space accessible from the surface (cf. closed-cell foam). There are many ways to test porosity in a substance or part, such as industrial CT scanning. The term porosity is used in multiple fields including pharmaceutics, ceramics, metallurgy, materials, manufacturing, petrophysics, hydrology, earth sciences, soil mechanics, and engineering. Void fraction in two-phase flow In gas-liquid two-phase flow, the void fraction is defined as the fraction of the flow-channel volume that is occupied by the gas phase or, alternatively, as the fraction of the cross-sectional area of the channel that is occupied by the gas phase. Void fraction usually varies from location to location in the fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marcasite
The mineral marcasite, sometimes called “white iron pyrite”, is iron sulfide (FeS2) with orthorhombic crystal structure. It is physically and crystallographically distinct from pyrite, which is iron sulfide with cubic crystal structure. Both structures do have in common that they contain the disulfide S22− ion, having a short bonding distance between the sulfur atoms. The structures differ in how these di-anions are arranged around the Fe2+ cations. Marcasite is lighter and more brittle than pyrite. Specimens of marcasite often crumble and break up due to the unstable crystal structure. On fresh surfaces, it is pale yellow to almost white and has a bright metallic luster. It tarnishes to a yellowish or brownish color and gives a black streak. It is a brittle material that cannot be scratched with a knife. The thin, flat, tabular crystals, when joined in groups, are called “cockscombs”. In marcasite jewellery, pyrite used as a gemstone is called “marcasite” ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrite
The mineral pyrite (), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral. Pyrite's metallic luster and pale brass-yellow hue give it a superficial resemblance to gold, hence the well-known nickname of ''fool's gold''. The color has also led to the nicknames ''brass'', ''brazzle'', and ''Brazil'', primarily used to refer to pyrite found in coal. The name ''pyrite'' is derived from the Greek (), 'stone or mineral which strikes fire', in turn from (), 'fire'. In ancient Roman times, this name was applied to several types of stone that would create sparks when struck against steel; Pliny the Elder described one of them as being brassy, almost certainly a reference to what we now call pyrite. By Georgius Agricola's time, , the term had become a generic term for all of the sulfide minerals. Pyrite is usually found associated with other sulfides or oxides in quartz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siderite
Siderite is a mineral composed of iron(II) carbonate (FeCO3). It takes its name from the Greek word σίδηρος ''sideros,'' "iron". It is a valuable iron mineral, since it is 48% iron and contains no sulfur or phosphorus. Zinc, magnesium and manganese commonly substitute for the iron resulting in the siderite- smithsonite, siderite-magnesite and siderite-rhodochrosite solid solution series. Siderite has Mohs hardness of 3.75-4.25, a specific gravity of 3.96, a white streak and a vitreous lustre or pearly luster. Siderite is antiferromagnetic below its Néel temperature of 37 K which can assist in its identification. It crystallizes in the trigonal crystal system, and are rhombohedral in shape, typically with curved and striated faces. It also occurs in masses. Color ranges from yellow to dark brown or black, the latter being due to the presence of manganese. Siderite is commonly found in hydrothermal veins, and is associated with barite, fluorite, galena, and oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German ''Calcit'', a term from the 19th century that came from the Latin word for lime, ''calx'' (genitive calcis) with the suffix "-ite" used to name minerals. It is thus etymologically related to chalk. When applied by archaeologists and stone trade professionals, the term alabaster is used not just as in geology and mineralogy, where it is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fossil
A fossil (from Classical Latin , ) is any preserved remains, impression, or trace of any once-living thing from a past geological age. Examples include bones, shells, exoskeletons, stone imprints of animals or microbes, objects preserved in amber, hair, petrified wood and DNA remnants. The totality of fossils is known as the ''fossil record''. Paleontology is the study of fossils: their age, method of formation, and evolutionary significance. Specimens are usually considered to be fossils if they are over 10,000 years old. The oldest fossils are around 3.48 billion years old to 4.1 billion years old. Early edition, published online before print. The observation in the 19th century that certain fossils were associated with certain rock strata led to the recognition of a geological timescale and the relative ages of different fossils. The development of radiometric dating techniques in the early 20th century allowed scientists to quantitatively measure the abs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |