|
List Of Gases
This is a list of gases at standard conditions, which means substances that boil or sublime at or below and 1 atm pressure and are reasonably stable. List This list is sorted by boiling point of gases in ascending order, but can be sorted on different values. "sub" and "triple" refer to the sublimation point and the triple point, which are given in the case of a substance that sublimes at 1 atm; "dec" refers to decomposition. "~" means approximately. Blue type items have an article available by clicking on the name. Known as gas The following list has substances known to be gases, but with an unknown boiling point. * Fluoroamine * Trifluoromethyl trifluoroethyl trioxide CF3OOOCF2CF3 boils between 10 and 20° * Bis-trifluoromethyl carbonate boils between −10 and +10° possibly +12, freezing −60° * Difluoroaminosulfinyl fluoride F2NS(O)F is a gas but decomposes over several hours * Trifluoromethylsulfinyl chloride CF3S(O)Cl * Nitrosyl cyanide ?−20° blue-green ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Oxygen In A Beaker 4
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly compressibility, incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to that of a solid, and much higher than that of a gas. Therefore, liquid and solid are classified as Condensed matter physics, condensed matter. Meanwhile, since both liquids and gases can flow, they are categorized as fluids. A liquid is composed of atoms or molecules held together by intermolecular bonds of intermediate strength. These forces allow the particles to move around one another while remaining closely packed. In contrast, solids have particles that are tightly bound by strong intermolecular forces, limiting their movement to small vibrations in fixed positions. Gases, on the other hand, consist of widely spaced, freely moving particles with only weak intermolecular forces. As temperature increases, the molecules in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Krypton
Krypton (from 'the hidden one') is a chemical element; it has symbol (chemistry), symbol Kr and atomic number 36. It is a colorless, odorless noble gas that occurs in trace element, trace amounts in the Earth's atmosphere, atmosphere and is often used with other rare gases in fluorescent lamps. Krypton is chemically inert. Krypton, like the other noble gases, is used in lighting and photography. Krypton light has many spectral lines, and krypton Plasma (physics), plasma is useful in bright, high-powered gas lasers (krypton ion laser, ion and excimer laser, excimer lasers), each of which resonates and amplifies a single spectral line. krypton fluoride laser, Krypton fluoride also makes a useful laser medium. From 1960 to 1983, the history of the metre#Krypton standard, official definition of the metre was based on the wavelength of one spectral line of krypton-86, because of the high power and relative ease of operation of krypton discharge tubes. History Krypton was discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Trifluoride
Boron trifluoride is the inorganic compound with the formula . This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds. Structure and bonding The geometry of a molecule of is Trigonal planar molecular geometry, trigonal planar. Its D3h symmetry group, symmetry conforms with the prediction of VSEPR theory. The molecule has no dipole moment by virtue of its high symmetry. The molecule is isoelectronic with the carbonate anion, . is commonly referred to as "electron deficiency, electron deficient," a description that is reinforced by its exothermic reactivity toward Lewis bases. In the boron trihalides, , the length of the B–X bonds (1.30 Å) is shorter than would be expected for single bonds, and this shortness may indicate stronger B–X pi bond, π-bonding in the fluoride. A facile explanation invokes the symmetry-allowed overlap of a p orbital on the boron atom with the in-phas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine Monofluoride
Chlorine monofluoride is a volatile interhalogen compound with the chemical formula . It is a colourless gas at room temperature and is stable even at high temperatures. When cooled to −100 °C, ClF condenses as a pale yellow liquid. Many of its properties are intermediate between its parent halogens, and . Geometry The molecular structure in the gas phase was determined by microwave spectroscopy; the bond length is ''r''e = 1.628341(4) Å. The bond length in the crystalline ClF is 1.628(1) Å; the lengthening relative to the free molecule is due to an interaction of the type F-Br···ClMe with a distance of 2.640(1) Å. In its molecular packing it shows very short intermolecular Cl···Cl contacts of 3.070(1) Å between neighboring molecules. Reactivity Chlorine monofluoride is a versatile fluorinating agent, converting metals and non-metals to their fluorides and releasing in the process. For example, it converts tungsten to tungsten he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Trifluoride
Phosphorus trifluoride (formula P F3), is a colorless and odorless gas. It is highly toxic and reacts slowly with water. Its main use is as a ligand in metal complexes. As a ligand, it parallels carbon monoxide in metal carbonyls, and indeed its toxicity is due to its binding with the iron in blood hemoglobin in a similar way to carbon monoxide. Physical properties Phosphorus trifluoride has an F−P−F bond angle of approximately 96.3°. Gaseous PF3 has a standard enthalpy of formation of −945 kJ/mol (−226 kcal/ mol). The phosphorus atom has a nuclear magnetic resonance chemical shift of 97 ppm (downfield of H3PO4). Properties Phosphorus trifluoride hydrolyzes especially at high pH, but it is less hydrolytically sensitive than phosphorus trichloride. It does not attack glass except at high temperatures, and anhydrous potassium hydroxide may be used to dry it with little loss. With hot metals, phosphides and fluorides are formed. With Lewis base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon double bonds). Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016) exceeds that of any other organic compound. Much of this production goes toward creating polyethylene, which is a widely used plastic containing polymer chains of ethylene units in various chain lengths. Production greenhouse gas emissions, emits greenhouse gases, including methane from feedstock production and carbon dioxide from any non-sustainable energy used. Ethylene is also an important natural plant hormone and is used in agriculture to induce ripening of fruits. The hydrate of ethylene is ethanol. Structure and properties This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized. Xenon is used in flash lamps and arc lamps, and as a general anesthetic. The first excimer laser design used a xenon dimer molecule (Xe2) as the lasing medium, and the earliest laser designs used xenon flash lamps as pumps. Xenon is also used to search for hypothetical weakly interacting massive particles and as a propellant for ion thrusters in spacecraft. Naturally occurring xenon consists of seven stable isotopes and two long-lived radioactive isotopes. More than 40 unstable xenon isotopes undergo radioactive decay, and the isotope ratios of xenon are an important tool for studying the early history of the Solar System. Radioactive xe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lower atmosphere to (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone's odor is reminiscent of chlorine, and detectable by many people at concentrations of as little as in air. Ozone's O3 chemical structure, structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetism, diamagnetic. At standard temperature and pressure, ozone is a pale blue gas that condenses at cryogenic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitrogen Difluoride
Dinitrogen difluoride is a chemical compound with the formula . It is a gas at room temperature, and was first identified in 1952 as the thermal decomposition product of the fluorine azide (). It has the structure and exists in both ''cis'' and ''trans'' isomers, as typical for diimides. Isomers The ''cis'' isomer has C2v symmetry and the ''trans'' isomer has C2h symmetry. These isomers can interconvert, but the process is slow enough at low temperature that the two can separated by low-temperature fractionation. The ''trans'' isomer is less thermodynamically stable but can be stored in glass vessels. The ''cis'' isomer attacks glass over a time scale of about 2 weeks to form silicon tetrafluoride and nitrous oxide: : Preparation Most preparations of dinitrogen difluoride give mixtures of the two isomers, but they can be prepared independently. An aqueous method involves ''N'',''N''-difluorourea with concentrated potassium hydroxide. This gives a 40% yield with three ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silane
Silane (Silicane) is an inorganic compound with chemical formula . It is a colorless, pyrophoric gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silanes with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. They are commonly used to apply coatings to surfaces or as an adhesion promoter. Production Commercial-scale routes Silane can be produced by several routes. Typically, it arises from the reaction of hydrogen chloride with magnesium silicide: : It is also prepared from metallurgical-grade silicon in a two-step process. First, silicon is treated with hydrogen chloride at about 300 °C to produce trichlorosilane, HSiCl3, along with hydrogen gas, according to the chemical equation : The trichlorosilane is then converted to a mixture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
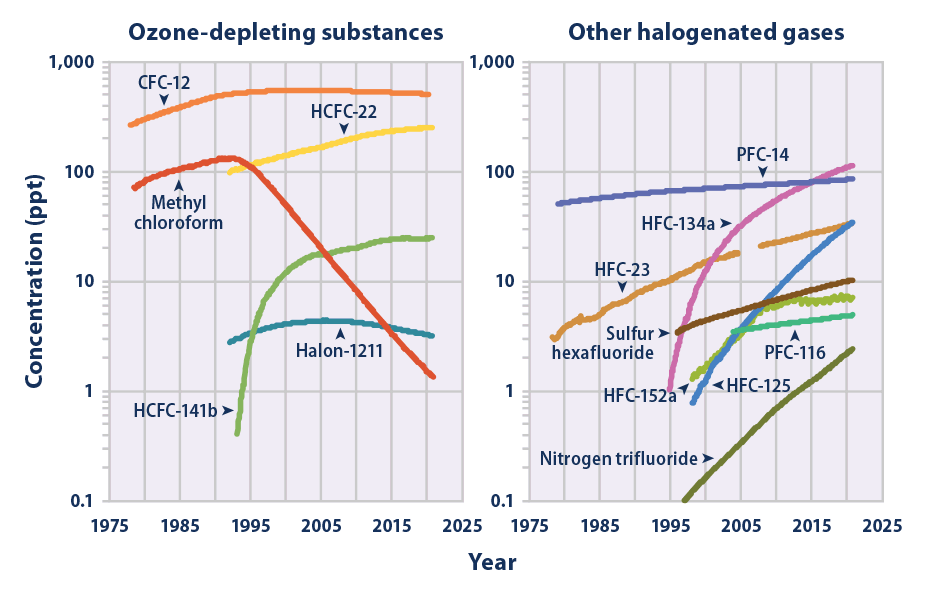
Nitrogen Trifluoride
Nitrogen trifluoride is the inorganic compound with the formula (). It is a colorless, non-flammable, toxic gas with a slightly musty odor. In contrast with ammonia, it is nonbasic. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. is a greenhouse gas, with a global warming potential (GWP) 17,200 times greater than that of when compared over a 100-year period. Synthesis and reactivity Nitrogen trifluoride can be prepared from the elements in the presence of an electric discharge. In 1903, Otto Ruff prepared nitrogen trifluoride by the electrolysis of a molten mixture of ammonium fluoride and hydrogen fluoride. It is far less reactive than the other nitrogen trihalides nitrogen trichloride, nitrogen tribromide, and nitrogen triiodide, all of which are explosive. Alone among the nitrogen trihalides it has a negative enthalpy of formation. It is prepared in modern times both by direct reaction of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |