|
Lantibiotic
Lantibiotics are a class of polycyclic peptide antibiotics that contain the characteristic thioether amino acids lanthionine or methyllanthionine, as well as the unsaturated amino acids dehydroalanine, and 2-aminoisobutyric acid. They belong to ribosomally synthesized and post-translationally modified peptides. Lanthionine is composed of two alanine residues that are crosslinked on their β-carbon atoms by a thioether (monosulfide) linkage. Lantibiotics are produced by a large number of Gram-positive bacteria such as ''Streptococcus'' and ''Streptomyces'' to attack other Gram-positive bacteria, and as such, they are considered a member of the bacteriocins. Bacteriocins are classified according to their extent of posttranslational modification. The lantibiotics are a class of more extensively modified bacteriocins, also called Class I bacteriocins. (Bacteriocins for which disulfide bonds are the only modification to the peptide are Class II bacteriocins.) Lantibiotics are we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribosomally Synthesized And Post-translationally Modified Peptides
Ribosomally synthesized and post-translationally modified peptides (RiPPs), also known as ribosomal natural products, are a diverse class of natural products of ribosomal origin. Consisting of more than 20 sub-classes, RiPPs are produced by a variety of organisms, including prokaryotes, eukaryotes, and archaea, and they possess a wide range of biological functions. As a consequence of the falling cost of genome sequencing and the accompanying rise in available genomic data, scientific interest in RiPPs has increased in the last few decades. Because the chemical structures of RiPPs are more closely predictable from genomic data than are other natural products (e.g. alkaloids, terpenoids), their presence in sequenced organisms can, in theory, be identified rapidly. This makes RiPPs an attractive target of modern natural product discovery efforts. Definition RiPPs consist of any peptides (i.e. molecular weight below 10 kDa) that are ribosomally-produced and undergo some degree ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteriocins
Bacteriocins are proteinaceous or peptidic toxins produced by bacteria to inhibit the growth of similar or closely related bacterial strain(s). They are similar to yeast and paramecium killing factors, and are structurally, functionally, and ecologically diverse. Applications of bacteriocins are being tested to assess their application as narrow-spectrum antibiotics. Bacteriocins were first discovered by André Gratia in 1925. He was involved in the process of searching for ways to kill bacteria, which also resulted in the development of antibiotics and the discovery of bacteriophage, all within a span of a few years. He called his first discovery a ''colicine'' because it killed ''E. coli.'' Classification Bacteriocins are categorized in several ways, including producing strain, common resistance mechanisms, and mechanism of killing. There are several large categories of bacteriocin which are only phenomenologically related. These include the bacteriocins from gram-posit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
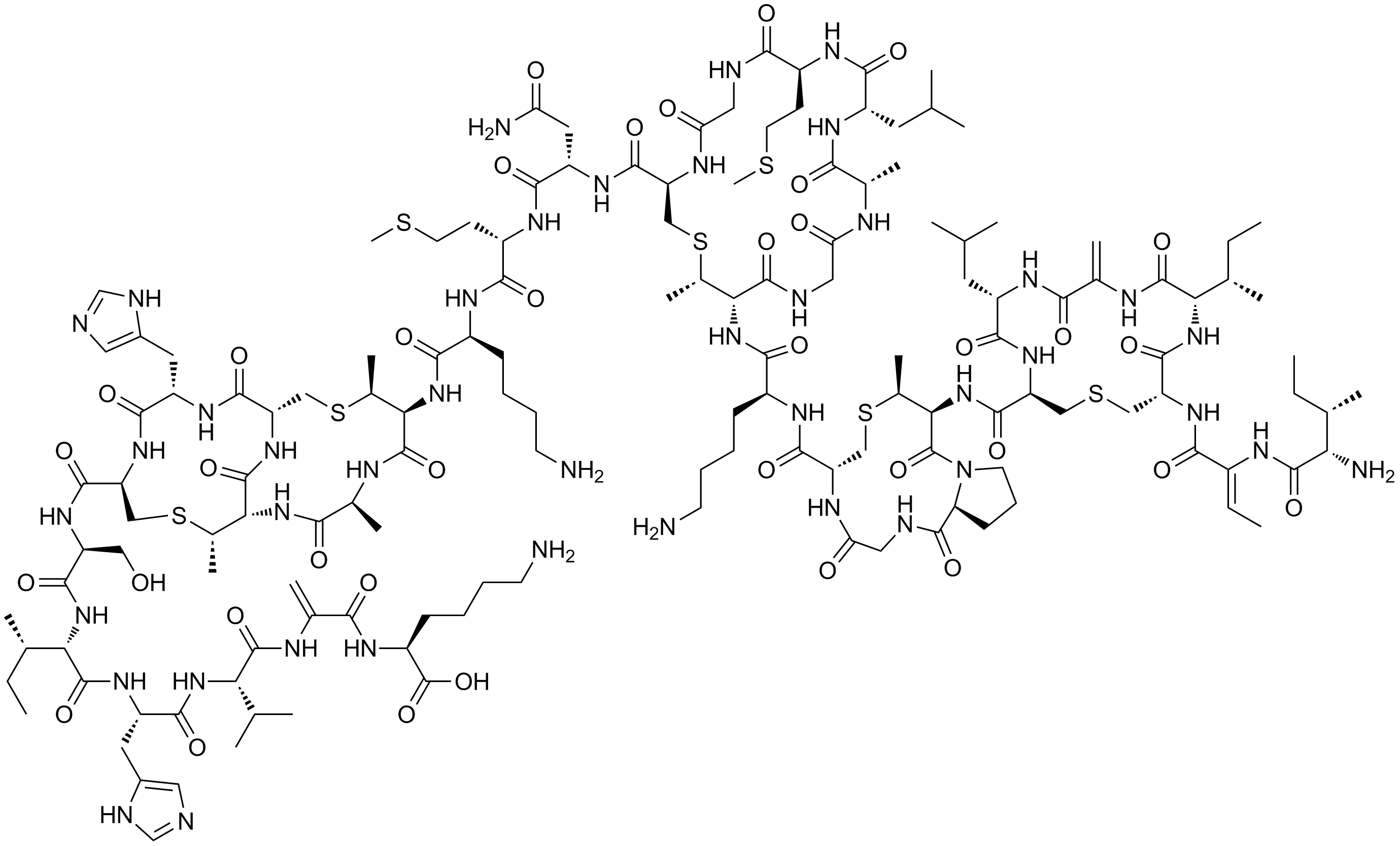
Bisin
Bisin is a naturally occurring lantibiotic (an antibacterial peptide) discovered by University of Minnesota microbiologist Dan O'Sullivan. Unlike earlier lantibiotics discovered, such as nisin, bisin also kills Gram-negative bacteria, including ''E. coli'', ''Salmonella ''Salmonella'' is a genus of rod-shaped (bacillus) Gram-negative bacteria of the family Enterobacteriaceae. The two species of ''Salmonella'' are ''Salmonella enterica'' and ''Salmonella bongori''. ''S. enterica'' is the type species and is fur ...'' and '' Listeria''. The food-preservative properties of bisin could lead to food products that resist spoilage for years. See also * Nisin - a related food preservative currently in use References {{Reflist External links Bactericidal Lanbiotic Inhibits Gram-negative Bacteria Office for Technology Commercialization, University of Minnesota. June 25, 2010. Lantibiotics Food preservation Peptides Bacteriocins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid II
Lipid II is a precursor molecule in the synthesis of the cell wall of bacteria. It is a peptidoglycan, which is amphipathic and named for its bactoprenol hydrocarbon chain, which acts as a lipid anchor, embedding itself in the bacterial cell membrane. Lipid II must translocate across the cell membrane to deliver and incorporate its disaccharide-pentapeptide "building block" into the peptidoglycan mesh. Lipid II is the target of several antibiotics. A number of analogous compounds are produced via a similar pathway in some bacteria, giving rise to cell wall modifications. See EC 2.4.1.227 for more information. Synthesis In peptidoglycan biosynthetic pathway Lipid II is the final intermediate in peptidoglycan synthesis. It is formed when the MurG transferase catalyzes addition of ''N''-acetylglucosamine (GlcNAc) to Lipid I, resulting in a complete disaccharide-pentapeptide monomer with a bactoprenol-pyrophosphate anchor. This occurs on the inside of the cytoplasmic membrane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthionine
Lanthionine is a nonproteinogenic amino acid with the chemical formula (HOOC-CH(NH2)-CH2-S-CH2-CH(NH2)-COOH). It is typically formed by a cysteine residue and a dehydrated serine residue. Despite its name, lanthionine does not contain the element lanthanum. Background In 1941, lanthionine was first isolated by treating wool with sodium carbonate. It was found to be a sulfur-containing amino acid; accordingly it was given the name lanthionine ool (Latin: ''Lana''), sulfur (Greek: ''theîon'') Lanthionine was first synthesized by alkylation of cysteine with β-chloroalanine. Lanthionines are found widely in nature. They have been isolated from human hair, lactalbumin, and feathers. Lanthionines have also been found in bacterial cell walls and are the components of a group of gene-encoded peptide antibiotics called lantibiotics, which includes nisin (a food preservative), subtilin, epidermin (effective against ''Staphylococcus'' and ''Streptococcus''), and ancovenin (an enzyme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nisin
Nisin is a polycyclic antibacterial peptide produced by the bacterium '' Lactococcus lactis'' that is used as a food preservative. It has 34 amino acid residues, including the uncommon amino acids lanthionine (Lan), methyllanthionine (MeLan), didehydroalanine (Dha), and didehydroaminobutyric acid (Dhb). These unusual amino acids are introduced by posttranslational modification of the precursor peptide. In these reactions a ribosomally synthesized 57-mer is converted to the final peptide. The unsaturated amino acids originate from serine and threonine, and the enzyme-catalysed addition of cysteine residues to the didehydro amino acids result in the multiple (5) thioether bridges. Subtilin and epidermin are related to nisin. All are members of a class of molecules known as lantibiotics. In the food industry, nisin is obtained from the culturing of ''L. lactis'' on natural substrates, such as milk or dextrose, and it is not chemically synthesized. It was originally isolated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Peptide
Cyclic peptides are polypeptide chains which contain a circular sequence of bonds. This can be through a connection between the amino and carboxyl ends of the peptide, for example in cyclosporin; a connection between the amino end and a side chain, for example in bacitracin; the carboxyl end and a side chain, for example in colistin; or two side chains or more complicated arrangements, for example in amanitin. Many cyclic peptides have been discovered in nature and many others have been synthesized in the laboratory. Their length ranges from just two amino acid residues to hundreds. In nature they are frequently antimicrobial or toxic; in medicine they have various applications, for example as antibiotics and immunosuppressive agents. Thin-Layer Chromatography (TLC) is a convenient method to detect cyclic peptides in crude extract from bio-mass. Classification Cyclic peptides can be classified according to the types of bonds that comprise the ring. *Homodetic cyclic peptides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Aminoisobutyric Acid
2-Aminoisobutyric acid (also known as α-aminoisobutyric acid, AIB, α-methylalanine, or 2-methylalanine) is the non-proteinogenic amino acid with the structural formula H2N-C(CH3)2-COOH. It is rare in nature, having been only found in meteorites, and some antibiotics of fungal origin, such as alamethicin and some lantibiotics. Synthesis In the laboratory, 2-aminoisobutyric acid may be prepared from acetone cyanohydrin, by reaction with ammonia followed by hydrolysis. Industrial scale synthesis can be achieved by the selective hydroamination of methacrylic acid. Biological activity 2-Aminoisobutyric acid is not one of the proteinogenic amino acids and is rather rare in nature (''cf.'' non-proteinogenic amino acids). It is a strong helix inducer in peptides due to Thorpe–Ingold effect of its gem-dimethyl group In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydroalanine
Dehydroalanine (Cα,β-didehydroalanine, α,β-di-dehydroalanine, 2-aminoacrylate, or 2,3-didehydroalanine) is a dehydroamino acid. It does not exist in its free form, but it occurs naturally as a residue found in peptides of microbial origin. As an amino acid residue, it is unusual because it has an unsaturated backbone. Structure and reactivity Like most primary enamines, dehydroalanine is unstable. Dehydroalanine hydrolyze to pyruvate. ''N''-Acylated derivatives of dehydroalanine, such as peptides and related compounds, are stable. For example, methyl 2-acetamidoacrylate is the N-acetylated derivative of the ester. As a residue in a peptide, it is generated by a post translational modification. The required precursors are serine or cysteine residues, which undergo enzyme-mediated loss of water and hydrogen sulfide, respectively. Most amino acid residues are unreactive toward nucleophiles, but those containing dehydroalanine or some other dehydroamino acids are exception ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid ''residues'' form the second-largest component ( water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotic Resistance
Antimicrobial resistance (AMR) occurs when microbes evolve mechanisms that protect them from the effects of antimicrobials. All classes of microbes can evolve resistance. Fungi evolve antifungal resistance. Viruses evolve antiviral resistance. Protozoa evolve antiprotozoal resistance, and bacteria evolve antibiotic resistance. Those bacteria that are considered extensively drug resistant (XDR) or totally drug-resistant (TDR) are sometimes called "superbugs".A.-P. Magiorakos, A. Srinivasan, R. B. Carey, Y. Carmeli, M. E. Falagas, C. G. Giske, S. Harbarth, J. F. Hinndler ''et al''Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria... Clinical Microbiology and Infection, Vol 8, Iss. 3 first published 27 July 2011 ia Wiley Online Library Retrieved 28 August 2020 Although antimicrobial resistance is a naturally-occurring process, it is often the result of improper usage of the drugs and management of the infections. Antibiotic resistance is a major su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |