dehydroalanine on:
[Wikipedia]
[Google]
[Amazon]
Dehydroalanine (Cα,β-didehydroalanine, α,β-di-dehydroalanine, 2-aminoacrylate, or 2,3-didehydroalanine) is a dehydroamino acid. It does not exist in its free form, but it occurs naturally as a residue found in
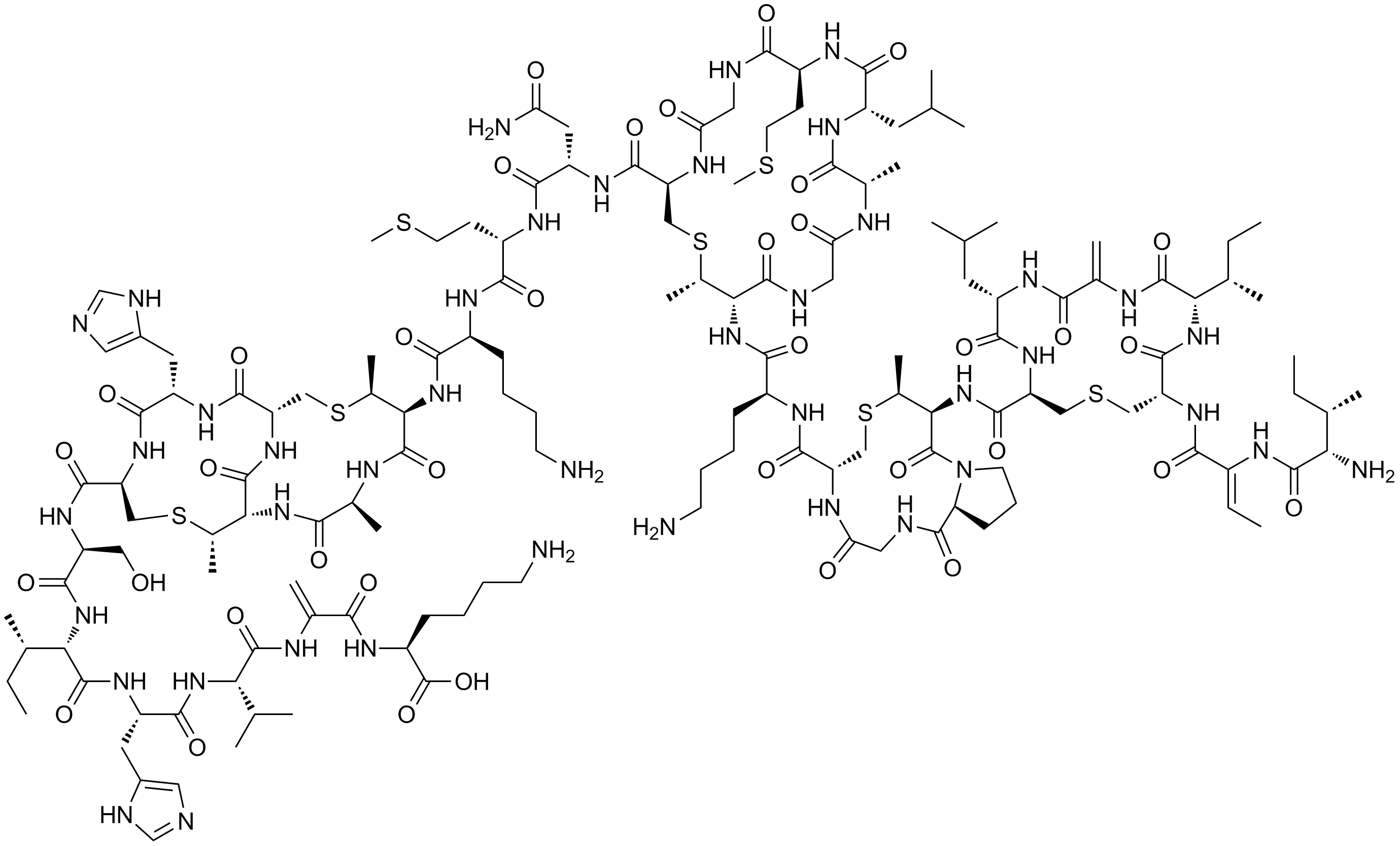 A dehydroalanine residue was long thought to be an important electrophilic catalytic residue in histidine ammonia-lyase and
A dehydroalanine residue was long thought to be an important electrophilic catalytic residue in histidine ammonia-lyase and
peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. ...
s of microbial
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
origin. As an amino acid residue, it is unusual because it has an unsaturated backbone.
Structure and reactivity
Like most primaryenamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
:
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and the ...
s, dehydroalanine is unstable. Dehydroalanine hydrolyze to pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic aci ...
.
''N''-Acylated derivatives of dehydroalanine, such as peptides and related compounds, are stable. For example, methyl 2-acetamidoacrylate
Methyl 2-acetamidoacrylate is the organic compound with the formula CH2=C(NHC(O)CH3)CO2CH3. It is the methyl ester of an ''N''-acetylacrylic acid, which in turn is a derivative of the unstable compound dehydroalanine. Acetylation of the amine in t ...
is the N-acetylated derivative of the ester. As a residue in a peptide, it is generated by a post translational modification. The required precursors are serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − for ...
or cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, s ...
residues, which undergo enzyme-mediated loss of water and hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The und ...
, respectively.
Most amino acid residues are unreactive toward nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
s, but those containing dehydroalanine or some other dehydroamino acids are exceptions. These are electrophilic due to the α,β-unsaturated carbonyl, and can, for example, alkylate other amino acids. This activity has made DHA useful synthetically to prepare lanthionine.
Occurrence
The dehydroalanine residue was first detected innisin
Nisin is a polycyclic antibacterial peptide produced by the bacterium '' Lactococcus lactis'' that is used as a food preservative. It has 34 amino acid residues, including the uncommon amino acids lanthionine (Lan), methyllanthionine (MeLan), d ...
, a cyclic peptide
Cyclic peptides are polypeptide chains which contain a circular sequence of bonds. This can be through a connection between the amino and carboxyl ends of the peptide, for example in cyclosporin; a connection between the amino end and a side ch ...
with antimicrobial activity. Dehydroalanine is also present in some lantibiotics
Lantibiotics are a class of polycyclic peptide antibiotics that contain the characteristic thioether amino acids lanthionine or methyllanthionine, as well as the unsaturated amino acids dehydroalanine, and 2-aminoisobutyric acid. They belong ...
and microcystin
Microcystins—or cyanoginosins—are a class of toxins produced by certain freshwater cyanobacteria, commonly known as blue-green algae. Over 250 different microcystins have been discovered so far, of which microcystin-LR is the most common. ...
s.
DHA can be formed from cysteine or serine by simple base catalysis without the need for an enzyme, which can happen during cooking and alkaline
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
food preparation processes. It can then alkylate other amino acid residues, such as lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated &minu ...
, forming lysinoalanine cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
s and racemization In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic (optically inactive) form. This creates a 1:1 molar ratio of enantiomers and is referred too as a racemic mixture (i.e. con ...
of the original alanine. The resulting proteins have lower nutritional quality for some species but higher nutritional quality for others. Some lysinoalanines may also cause kidney enlargement in rats.
Many dehydroalanine-containing peptides are toxic.
 A dehydroalanine residue was long thought to be an important electrophilic catalytic residue in histidine ammonia-lyase and
A dehydroalanine residue was long thought to be an important electrophilic catalytic residue in histidine ammonia-lyase and phenylalanine ammonia-lyase
The enzyme phenylalanine ammonia lyase (EC 4.3.1.24) catalyzes the conversion of L-phenylalanine to ammonia and ''trans''-cinnamic acid.:
:L-phenylalanine = ''trans''-cinnamate + NH3
Phenylalanine ammonia lyase (PAL) is the first and committed ...
enzymes, but the active residue was later found instead to be a different unsaturated alanine derivative — 3,5-dihydro-5-methyldiene-4''H''-imidazol-4-one — that is even more electrophilic.{{cite journal , vauthors = Calabrese JC, Jordan DB, Boodhoo A, Sariaslani S, Vannelli T , title = Crystal structure of phenylalanine ammonia lyase: multiple helix dipoles implicated in catalysis , journal = Biochemistry , volume = 43 , issue = 36 , pages = 11403–16 , date = September 2004 , pmid = 15350127 , doi = 10.1021/bi049053+
References