|
Lability
Lability refers to the degree that something is likely to undergo change. It is the opposite ( antonym) of stability. Biochemistry In reference to biochemistry, this is an important concept as far as kinetics is concerned in metalloproteins. This can allow for the rapid synthesis and degradation of substrates in biological systems. Biology Cells Labile cells refer to cells that constantly divide by entering and remaining in the cell cycle. These are contrasted with "stable cells" and "permanent cells". An important example of this is in the epithelium of the cornea, where cells divide at the basal level and move upwards, and the topmost cells die and fall off. Proteins In medicine, the term "labile" means susceptible to alteration or destruction. For example, a heat-labile protein is one that can be changed or destroyed at high temperatures. The opposite of labile in this context is "stable". Soils Compounds or materials that are easily transformed (often by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emotional Lability
In medicine and psychology, emotional lability is a Medical sign, sign or symptom typified by exaggerated changes in mood or affect (psychology), affect in quick succession. Sometimes the emotions expressed outwardly are very different from how the person feels on the inside. These strong emotions can be a disproportionate response to something that happened, but other times there might be no trigger at all. The person experiencing emotional lability usually feels like they do not have control over their emotions. For example, someone might cry uncontrollably in response to any strong emotion even if they do not feel sad or unhappy. Emotional lability is seen or reported in various conditions including borderline personality disorder, histrionic personality disorder, post-traumatic stress disorder, hypomanic or manic episodes of bipolar disorder, and neurological disorders or brain injury (where it is termed pseudobulbar affect), such as after a stroke. It has sometimes been found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cornea
The cornea is the transparency (optics), transparent front part of the eyeball which covers the Iris (anatomy), iris, pupil, and Anterior chamber of eyeball, anterior chamber. Along with the anterior chamber and Lens (anatomy), lens, the cornea Refraction, refracts light, accounting for approximately two-thirds of the eye's total optical power. In humans, the refractive power of the cornea is approximately 43 dioptres. The cornea can be reshaped by surgical procedures such as LASIK. While the cornea contributes most of the eye's focusing power, its Focus (optics), focus is fixed. Accommodation (eye), Accommodation (the refocusing of light to better view near objects) is accomplished by changing the geometry of the lens. Medical terms related to the cornea often start with the prefix "''wikt:kerat-, kerat-''" from the Ancient Greek, Greek word κέρας, ''horn''. Structure The cornea has myelinated, unmyelinated nerve endings sensitive to touch, temperature and chemicals; a to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins. Abundance It is estimated that approximately half of all proteins contain a metal. In another estimate, about one quarter to one third of all proteins are proposed to require metals to carry out their functions. Thus, metalloproteins have many different functions in cells, such as storage and transport of proteins, enzymes and signal transduction proteins, or infectious diseases. The abundance of metal binding proteins may be inherent to the amino acids that proteins use, as even artificial proteins without evolutionary history will readily bind metals. Most metals in the human body are bound to proteins. For instance, the relatively high concentration of iron in the human body ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Cycle
The cell cycle, or cell-division cycle, is the sequential series of events that take place in a cell (biology), cell that causes it to divide into two daughter cells. These events include the growth of the cell, duplication of its DNA (DNA replication) and some of its organelles, and subsequently the partitioning of its cytoplasm, chromosomes and other components into two daughter cells in a process called cell division. In eukaryotic cells (having a cell nucleus) including animal, plant, fungal, and protist cells, the cell cycle is divided into two main stages: interphase, and the M phase that includes mitosis and cytokinesis. During interphase, the cell grows, accumulating nutrients needed for mitosis, and replicates its DNA and some of its organelles. During the M phase, the replicated Chromosome, chromosomes, organelles, and cytoplasm separate into two new daughter cells. To ensure the proper replication of cellular components and division, there are control mechanisms kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biological Activity
In pharmacology, biological activity or pharmacological activity describes the beneficial or adverse effects of a drug on living matter. When a drug is a complex chemical mixture, this activity is exerted by the substance's active ingredient or pharmacophore but can be modified by the other constituents. Among the various properties of chemical compounds, pharmacological/biological activity plays a crucial role since it suggests uses of the compounds in the medical applications. However, chemical compounds may show some adverse and toxic effects which may prevent their use in medical practice. Biological activity is usually measured by a bioassay and the activity is generally dosage-dependent, which is investigated via dose-response curves. Further, it is common to have effects ranging from beneficial to adverse for one substance when going from low to high doses. Activity depends critically on fulfillment of the ADME criteria. To be an effective drug, a compound not only ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
Phosphates are the naturally occurring form of the element phosphorus. In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, phosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one proton gives the dihydrogen phosphate ion while removal of two protons gives the hydrogen phosphate ion . These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate or orthophosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solution (chemistry)
In chemistry, a solution is defined by IUPAC as "A liquid or solid phase containing more than one substance, when for convenience one (or more) substance, which is called the solvent, is treated differently from the other substances, which are called solutes. When, as is often but not necessarily the case, the sum of the mole fractions of solutes is small compared with unity, the solution is called a dilute solution. A superscript attached to the ∞ symbol for a property of a solution denotes the property in the limit of infinite dilution." One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term " aqueous solution" is used when one of the solvents is water. Types ''Homogeneous'' means that the components of the mixture form a single phase. ''Heterogeneous'' means that the components of the mixture are of different phase. The properties of the mixture (such as concentration, temp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
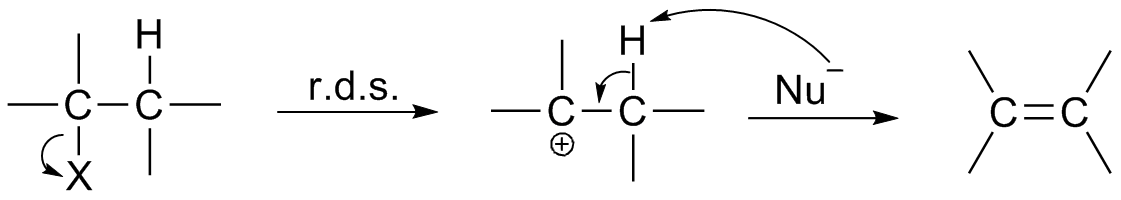
Reaction Intermediate
In chemistry, a reaction intermediate, or intermediate, is a molecular entity arising within the sequence of a stepwise chemical reaction. It is formed as the reaction product of an elementary step, from the reactants and/or preceding intermediates, but is consumed in a later step. It does not appear in the chemical equation for the overall reaction. For example, consider this hypothetical reaction: :A + B → C + D If this overall reaction comprises two elementary steps thus: :A + B → X :X → C + D then X is a reaction intermediate. The phrase ''reaction intermediate'' is often abbreviated to the single word ''intermediate'', and this is IUPAC's preferred form of the term. But this shorter form has other uses. It often refers to reactive intermediates. It is also used more widely for chemicals such as cumene which are traded within the chemical industry but are not generally of value outside it. IUPAC definition The IUPAC Gold Book defines an ''intermediate'' as a co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metastability
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy. A ball resting in a hollow on a slope is a simple example of metastability. If the ball is only slightly pushed, it will settle back into its hollow, but a stronger push may start the ball rolling down the slope. Bowling pins show similar metastability by either merely wobbling for a moment or tipping over completely. A common example of metastability in science is isomerisation. Higher energy isomers are long lived because they are prevented from rearranging to their preferred ground state by (possibly large) barriers in the potential energy. During a metastable state of finite lifetime, all state-describing parameters reach and hold stationary values. In isolation: *the state of least energy is the only one the system will inhabit for an indefinite length of time, until more external energy is added to the system (unique "absolu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Instability
In dynamical systems instability means that some of the outputs or internal states increase with time, without bounds. Not all systems that are not stable are unstable; systems can also be marginally stable or exhibit limit cycle behavior. In structural engineering, a structural beam or column can become unstable when excessive compressive load is applied. Beyond a certain threshold, structural deflections magnify stresses, which in turn increases deflections. This can take the form of buckling or crippling. The general field of study is called structural stability. Atmospheric instability is a major component of all weather systems on Earth. Instability in control systems In the theory of dynamical systems, a state variable in a system is said to be unstable if it evolves without bounds. A system itself is said to be unstable if at least one of its state variables is unstable. In continuous time control theory, a system is unstable if any of the roots of its charac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynamic Equilibrium
In chemistry, a dynamic equilibrium exists once a reversible reaction occurs. Substances initially transition between the reactants and products at different rates until the forward and backward reaction rates eventually equalize, meaning there is no net change. Reactants and products are formed at such a rate that the concentration of neither changes. It is a particular example of a system in a steady state. Examples In a new bottle of soda, the concentration of carbon dioxide in the liquid phase has a particular value. If half of the liquid is poured out and the bottle is sealed, carbon dioxide will leave the liquid phase at an ever-decreasing rate, and the partial pressure of carbon dioxide in the gas phase will increase until equilibrium is reached. At that point, due to thermal motion, a molecule of CO2 may leave the liquid phase, but within a very short time another molecule of CO2 will pass from the gas to the liquid, and vice versa. At equilibrium, the rate of tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |