|
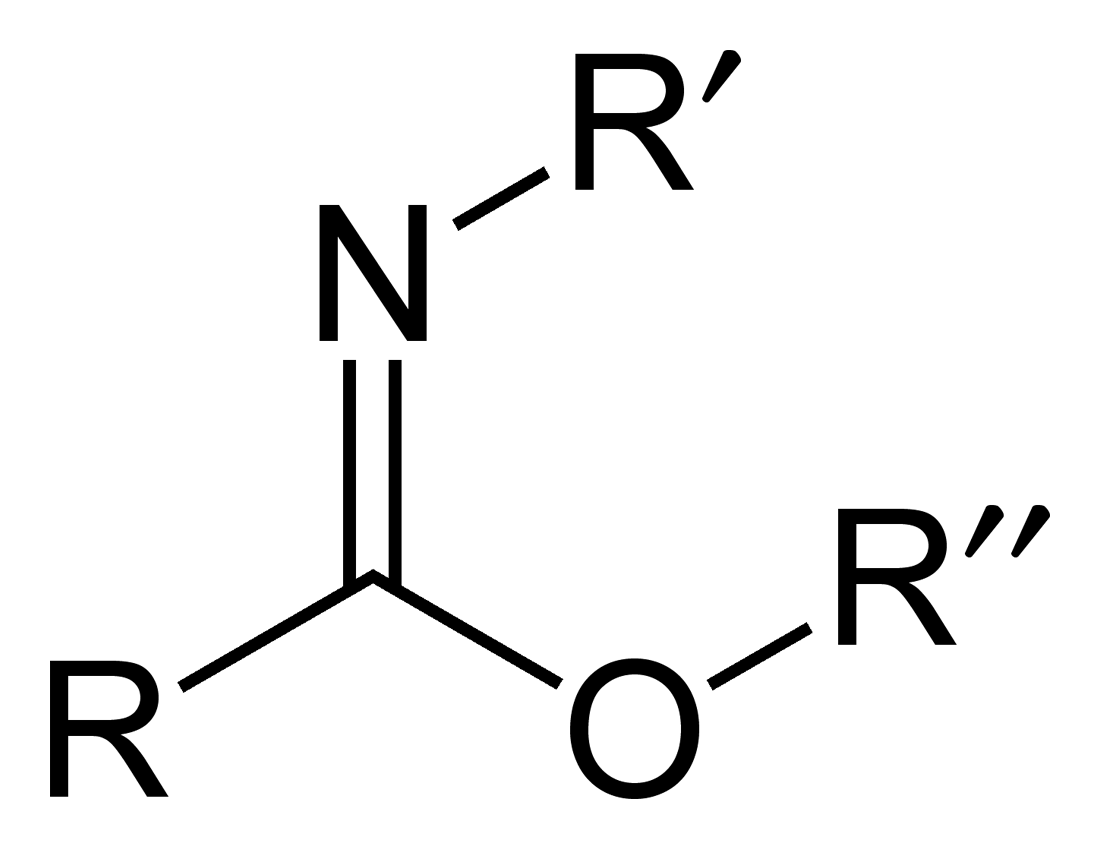
Iminoether
Carboximidates (or more general imidates) are organic compounds, which can be thought of as esters formed between an imidic acid () and an alcohol, with the general formula . They are also known as imino ethers, since they resemble imines () with an oxygen atom connected to the carbon atom of the C=N double bond. Synthesis Imidates may be generated by a number of synthetic routes, but are in general formed by the Pinner reaction. This proceeds via the acid catalyzed attack of nitriles by alcohols. Imidates produced in this manner are formed as their hydrochloride salts, which are sometimes referred to as Pinner salts. Carboximidates are also formed as intermediates in the Mumm rearrangement and the Overman rearrangement. Imidate/amidate anions An amidate/imidate anion is formed upon deprotonation of an amide or imidic acid. Since amides and imidic acids are tautomers, they form the same anion upon deprotonation. The two names are thus synonyms describing the same anion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfaction, olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of Resonance (chemistry), resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-covalent bond, bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Friedrich August Kekulé ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichloroacetonitrile
Trichloroacetonitrile is an organic compound with the formula CCl3CN. It is a colourless liquid, although commercial samples often are brownish. It is used commercially as a precursor to the fungicide etridiazole. It is prepared by dehydration of trichloroacetamide. As a bifunctional compound, trichloroacetonitrile can react at both the trichloromethyl and the nitrile group. The Electrophilic aromatic directing groups, electron-withdrawing effect of the trichloromethyl group activates the nitrile group for nucleophilic additions. The high Reactivity (chemistry), reactivity makes trichloroacetonitrile a versatile reagent, but also causes its susceptibility towards hydrolysis. Synthesis The production of trichloroacetonitrile by dehydration of trichloroacetamide was first described in 1873 by L. Bisschopinck at the KU Leuven, Katholieke Universiteit Leuven. : Trichloroacetonitrile can be obtained by Chlorination reaction, chlorination of acetonitrile on a zinc, copper and alkali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzyl Alcohol
Benzyl alcohol (also known as α-cresol) is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is useful as a solvent for its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide. Natural occurrences Benzyl alcohol is produced naturally by many plants and is commonly found in fruits and teas. It is also found in a variety of essential oils including jasmine, hyacinth and ylang-ylang. It is also found in castoreum from the castor sacs of beavers. Benzyl esters also occur naturally. Preparation Benzyl alcohol is produced industrially from toluene via ben ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protecting Group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis. In many preparations of delicate organic compounds, specific parts of the molecules cannot survive the required reagents or chemical environments. These parts (functional groups) must be protected. For example, lithium aluminium hydride is a highly reactive reagent that usefully reduces esters to alcohols. It always reacts with carbonyl groups, and cannot be discouraged by any means. When an ester must be reduced in the presence of a carbonyl, hydride attack on the carbonyl must be prevented. One way to do so converts the carbonyl into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the hydride step is complete, aqueous acid removes the acetal, restoring the carbonyl. This step ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzyl 2,2,2-trichloroacetimidate
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group (). Nomenclature In IUPAC nomenclature, the prefix benzyl refers to a substituent, for example benzyl chloride or benzyl benzoate. Benzyl is not to be confused with phenyl with the formula . The term benzylic is used to describe the position of the first carbon bonded to a benzene or other aromatic ring. For example, is referred to as a "benzylic" carbocation. The benzyl free radical has the formula . The benzyl cation or phenylcarbenium ion is the carbocation with formula ; the benzyl anion or phenylmethanide ion is the carbanion with the formula . None of these species can be formed in significant amounts in the solution phase under normal conditions, but they are useful referents for discussion of reaction mechanisms and may exist as reactive intermediates. Abbreviations Benzyl is most commonly abbreviated Bn. Fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chapman Rearrangement
Carboximidates (or more general imidates) are organic compounds, which can be thought of as esters formed between an imidic acid () and an alcohol, with the general formula . They are also known as imino ethers, since they resemble imines () with an oxygen atom connected to the carbon atom of the C=N double bond. Synthesis Imidates may be generated by a number of synthetic routes, but are in general formed by the Pinner reaction. This proceeds via the acid catalyzed attack of nitriles by alcohols. Imidates produced in this manner are formed as their hydrochloride salts, which are sometimes referred to as Pinner salts. Carboximidates are also formed as intermediates in the Mumm rearrangement and the Overman rearrangement. Imidate/amidate anions An amidate/imidate anion is formed upon deprotonation of an amide or imidic acid. Since amides and imidic acids are tautomers, they form the same anion upon deprotonation. The two names are thus synonyms describing the same anion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Newman–Kwart Rearrangement
The Newman–Kwart rearrangement is a type of rearrangement reaction in which the aryl group of an ''O''-aryl thiocarbamate, ArOC(=S)NMe2, migrates from the oxygen atom to the sulfur atom, forming an ''S''-aryl thiocarbamate, ArSC(=O)NMe2. The reaction is named after its discoverers, Melvin Spencer Newman and Harold Kwart. The reaction is a manifestation of the double bond rule. The Newman–Kwart reaction represents a useful synthetic tool for the preparation of thiophenol derivatives. : Mechanism The Newman–Kwart rearrangement is intramolecular. It is generally believed to be a concerted process, proceeding ''via'' a four-membered cyclic transition state (rather than a two-step process passing through a discrete reactive intermediate). The enthalpy of activation for this transition state is generally quite high for typical substrates (Δ''H''‡ ~ 30 to 40 kcal/mol), necessitating high reaction temperatures (200 to 300 °C, Ph2O as solvent or heat). : A Pd-catalyzed pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arthur William Chapman
Arthur is a masculine given name of uncertain etymology. Its popularity derives from it being the name of the legendary hero King Arthur. A common spelling variant used in many Slavic, Romance, and Germanic languages is Artur. In Spanish and Italian it is Arturo. Etymology The earliest attestation of the name Arthur is in the early 9th century Welsh-Latin text ''Historia Brittonum'', where it refers to a circa 5th century Romano-British general who fought against the invading Saxons, and who later gave rise to the famous King Arthur of medieval legend and literature. A possible earlier mention of the same man is to be found in the epic Welsh poem ''Y Gododdin'' by Aneirin, which some scholars assign to the late 6th century, though this is still a matter of debate and the poem only survives in a late 13th century manuscript entitled the Book of Aneirin. A 9th-century Breton landowner named Arthur witnessed several charters collected in the '' Cartulary of Redon''. The Irish borrow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels–Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic chemistry has a strong tradition of naming a specif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pinner Übersicht Version 2
Pinner is a suburb in the London Borough of Harrow, northwest London, England, northwest of Charing Cross, close to the border with London Borough of Hillingdon, Hillingdon, historically in the county of Middlesex. The population was 38,698 in 2021. Originally a mediaeval hamlet (place), hamlet, the St John the Baptist, Pinner, St John Baptist church dates from the 14th century and other parts of the historic village include Tudor period, Tudor buildings. The newer High Street is mainly 18th-century buildings, while Bridge Street has a more urban character and many chain stores. History Pinner was originally a Hamlet (place), hamlet, first recorded in 1231 as ''Pinnora'', although the already archaic ''-ora'' (meaning 'hill') suggests its origins lie no later than circa 900. The name ''Pinn'' is shared with the River Pinn, which runs through the middle of Pinner. Another suggestion of the name is that it means 'hill-slope shaped like a pin'. The oldest part of the town lies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |