|
IPWR-900
The Indian Pressurized Water Reactor-900 (IPWR-900) is a class of pressurized water reactors being designed by Bhabha Atomic Research Centre (BARC) in partnership with Nuclear Power Corporation of India Limited to supplement the Indian three-stage nuclear power programme History BARC has developed a 83 MW compact light water reactor known as CLWR-B1 for the Indian Navy's Arihant-class submarine program which includes a prototype reactor operating at Kalpakkam since 2002 and was made operational in the INS Arihant in 2013. The experience gained in the naval reactor program is being used to develop a commercial electricity generation reactor of 900 MWe capacity. To support the industrial capacity to fabricate the large forgings for a reactor pressure vessel, a heavy forge unit has been set up as a joint venture by the Nuclear Power Corporation of India Limited and Indian engineering conglomerate Larsen & Toubro's subsidiary L&T Special Steels and Heavy Forgings Limited in Hazi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pressurized Water Reactor
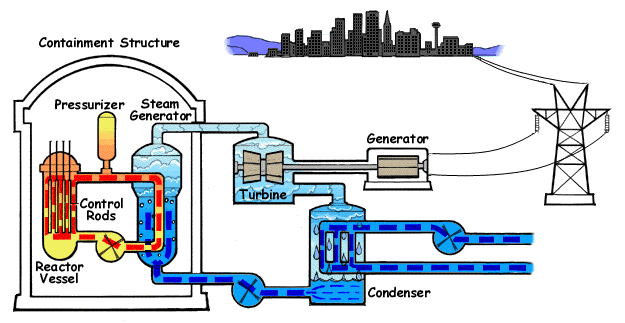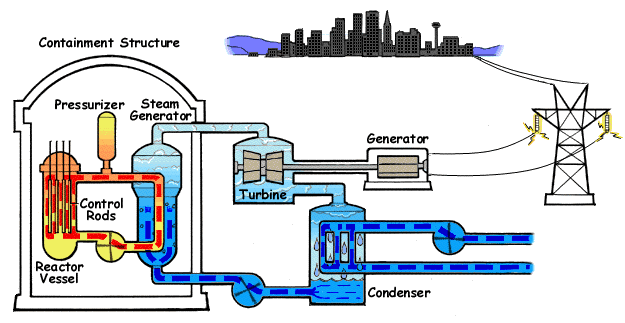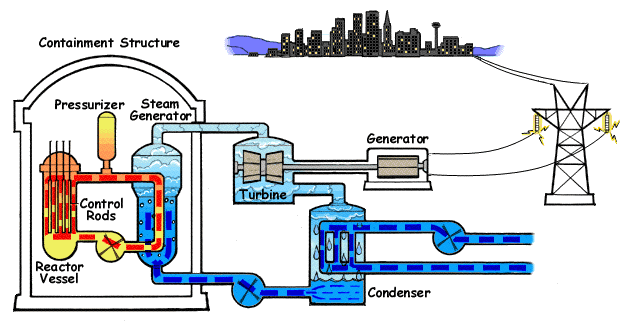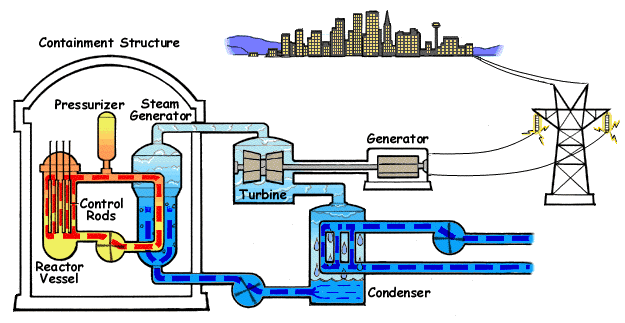
A pressurized water reactor (PWR) is a type of light-water nuclear reactor. PWRs constitute the large majority of the world's nuclear power plants (with notable exceptions being the UK, Japan and Canada). In a PWR, the primary coolant (water) is pumped under high pressure to the reactor core where it is heated by the energy released by the fission of atoms. The heated, high pressure water then flows to a steam generator, where it transfers its thermal energy to lower pressure water of a secondary system where steam is generated. The steam then drives turbines, which spin an electric generator. In contrast to a boiling water reactor (BWR), pressure in the primary coolant loop prevents the water from boiling within the reactor. All light-water reactors use ordinary water as both coolant and neutron moderator. Most use anywhere from two to four vertically mounted steam generators; VVER reactors use horizontal steam generators. PWRs were originally designed to serve as nuclear marine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bhabha Atomic Research Centre
The Bhabha Atomic Research Centre (BARC) is India's premier nuclear research facility, headquartered in Trombay, Mumbai, Maharashtra, India. It was founded by Homi Jehangir Bhabha as the Atomic Energy Establishment, Trombay (AEET) in January 1954 as a multidisciplinary research program essential for India's nuclear program. It operates under the Department of Atomic Energy (DAE), which is directly overseen by the Prime Minister of India. BARC is a multi-disciplinary research centre with extensive infrastructure for advanced research and development covering the entire spectrum of nuclear science, chemical engineering, material sciences and metallurgy, electronic instrumentation, biology and medicine, supercomputing, high-energy physics and plasma physics and associated research for Indian nuclear programme and related areas. BARC's core mandate is to sustain peaceful applications of nuclear energy. It manages all facets of nuclear power generation, from the theoretica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
India's Three-stage Nuclear Power Programme
India's three-stage nuclear power programme was formulated by Homi Bhabha, the well-known physicist, in the 1950s to secure the country's long term energy independence, through the use of uranium and thorium reserves found in the monazite sands of coastal regions of South India. The ultimate focus of the programme is on enabling the thorium reserves of India to be utilised in meeting the country's energy requirements. Thorium is particularly attractive for India, as India has only around 1–2% of the global uranium reserves, but one of the largest shares of global thorium reserves at about 25% of the world's known thorium reserves. However, thorium is more difficult to use than uranium as a fuel because it requires breeding, and global uranium prices remain low enough that breeding is not cost effective. India published about twice the number of papers on thorium as its nearest competitors, during each of the years from 2002 to 2006. The Indian nuclear establishment estimates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Absorber
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable effect. However, neutron-absorbing materials, also called poisons, are intentionally inserted into some types of reactors in order to lower the high reactivity of their initial fresh fuel load. Some of these poisons deplete as they absorb neutrons during reactor operation, while others remain relatively constant. The capture of neutrons by short half-life fission products is known as reactor poisoning; neutron capture by long-lived or stable fission products is called reactor slagging. Transient fission product poisons Some of the fission products generated during nuclear reactions have a high neutron absorption capacity, such as xenon-135 (microscopic cross-section σ = 2,000,000 barns (b); up to 3 million barns in reactor c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gadolinium(III) Oxide
Gadolinium(III) oxide (archaically gadolinia) is an inorganic compound with the formula Gd2O3. It is one of the most commonly available forms of the rare-earth element gadolinium, derivatives of which are potential contrast agents for magnetic resonance imaging. Structure Gadolinium oxide adopts two structures. The cubic ( cI80, Ia), No. 206) structure is similar to that of manganese(III) oxide and heavy trivalent lanthanide sesquioxides. The cubic structure features two types of gadolinium sites, each with a coordination number of 6 but with different coordination geometries. The second polymorph is monoclinic (Pearson symbol mS30, space group C2/m, No. 12). At room temperature, the cubic structure is more stable. The phase change to the monoclinic structure takes place at 1200 °C. Above 2100 °C to the melting point at 2420 °C, a hexagonal phase dominates. Preparation and chemistry Gadolinium oxide can be formed by thermal decomposition of the hydroxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen or moisture slowly to form a black coating. Gadolinium below its Curie point of is ferromagnetic, with an attraction to a magnetic field higher than that of nickel. Above this temperature it is the most paramagnetic element. It is found in nature only in an oxidized form. When separated, it usually has impurities of the other rare-earths because of their similar chemical properties. Gadolinium was discovered in 1880 by Jean Charles de Marignac, who detected its oxide by using spectroscopy. It is named after the mineral gadolinite, one of the minerals in which gadolinium is found, itself named for the Finnish chemist Johan Gadolin. Pure gadolinium was first isolated by the chemist Paul-Émile Lecoq de Boisbaudran around 1886. Gadolini ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine Pit
The iodine pit, also called the iodine hole or xenon pit, is a temporary disabling of a nuclear reactor due to buildup of short- lived nuclear poisons in the reactor core. The main isotope responsible is 135Xe, mainly produced by natural decay of 135I. 135I is a weak neutron absorber, while 135Xe is the strongest known neutron absorber. When 135Xe builds up in the fuel rods of a reactor, it significantly lowers their reactivity, by absorbing a significant amount of the neutrons that provide the nuclear reaction. The presence of 135I and 135Xe in the reactor is one of the main reasons for its power fluctuations in reaction to change of control rod positions. The buildup of short-lived fission products acting as nuclear poisons is called reactor poisoning, or xenon poisoning. Buildup of stable or long-lived neutron poisons is called reactor slagging. Fission products decay and burnup One of the common fission products is 135Te, which undergoes beta decay with half-life of 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Void Coefficient
In nuclear engineering, the void coefficient (more properly called void coefficient of reactivity) is a number that can be used to estimate how much the reactivity of a nuclear reactor changes as voids (typically steam bubbles) form in the reactor moderator or coolant. Net reactivity in a reactor is the sum total of all these contributions, of which the void coefficient is but one. Reactors in which either the moderator or the coolant is a liquid typically will have a void coefficient value that is either negative (if the reactor is under-moderated) or positive (if the reactor is over-moderated). Reactors in which neither the moderator nor the coolant is a liquid (e.g., a graphite-moderated, gas-cooled reactor) will have a void coefficient value equal to zero. It is unclear how the definition of 'void' coefficient applies to reactors in which the moderator/coolant is neither liquid nor gas (supercritical water reactor). Explanation Nuclear fission reactors run on nuclear chain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Control Rod
Control rods are used in nuclear reactors to control the rate of fission of the nuclear fuel – uranium or plutonium. Their compositions include chemical elements such as boron, cadmium, silver, hafnium, or indium, that are capable of absorbing many neutrons without themselves decaying. These elements have different neutron capture cross sections for neutrons of various energies. Boiling water reactors (BWR), pressurized water reactors (PWR), and heavy-water reactors (HWR) operate with thermal neutrons, while breeder reactors operate with fast neutrons. Each reactor design can use different control rod materials based on the energy spectrum of its neutrons. Control rods have been used in nuclear aircraft engines like Project Pluto as a method of control. Operating principle Control rods are inserted into the core of a nuclear reactor and adjusted in order to control the rate of the nuclear chain reaction and, thereby, the thermal power output of the reactor, the rate o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insoluble to water, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at a price of $13.2 billion. Structure In all three of its main dioxides, titanium exhibits octahedral geometry, being bonded to six oxide anions. The oxides in turn are bonded to three Ti centers. The overall crystal structure of rutile is tetragonal in symmetry whereas anatase and brookite are orthorhombic. The oxygen substructures are all slight dist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dysprosium(III) Oxide
Dysprosium oxide (Dy2O3) is a sesquioxide compound of the rare earth metal dysprosium. It is a pastel yellowish-greenish, slightly hygroscopic powder having specialized uses in ceramics, glass, phosphors, lasers, as a Faraday rotator A Faraday rotator is a polarization rotator based on the Faraday effect, a magneto-optic effect involving transmission of light through a material when a longitudinal static magnetic field is present. The state of polarization (such as the axis o ... and dysprosium metal halide lamps. It can react with acids to produce the corresponding dysprosium(III) salts: :Dy2O3 + 6 HCl → 2 DyCl3 + 3 H2O References Dysprosium compounds Sesquioxides {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |