|
Hofmeister Series
The Hofmeister series or lyotropic series is a classification of ions in order of their lyotrophic properties, which is the ability to salt out or salt in proteins. The effects of these changes were first worked out by Franz Hofmeister, who studied the effects of cations and anions on the solubility of proteins. Kosmotropes and chaotropes Highly charged ions interact strongly with water, breaking hydrogen bonds and inducing electrostatic structuring of nearby water, and are thus called "structure-makers" or " kosmotropes". Conversely, weak ions can disrupt the structure of water, and are thus called "structure-breakers" or " chaotropes". The order of the tendency of ions to make or break water structure is the basis of the Hofmeister series. Hofmeister discovered a series of salts that have consistent effects on the solubility of proteins and, as it was discovered later, on the stability of their secondary and tertiary structures. Anions appear to have a larger eff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prag Medizinische Fakultät Franz Hofmeister 448
Prag may refer to: * German, Swedish, Danish, Icelandic and Turkish for Prague * Adi Prag (born 1957), Israeli Olympic swimmer *Derek Prag (1923–2010), British politician *Rameshbabu Praggnanandhaa (born 2005), Indian chess player * Prague (2006 film), ''Prague'' (2006 film), Danish film starring Mads Mikkelsen, Stine Stengade and Jana Plodková {{disambiguation, surname ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Science (journal)
''Chemical Science'' is a weekly peer-reviewed scientific journal covering all aspects of chemistry. It is the flagship journal of the Royal Society of Chemistry. It was established in July 2010 and is published by the Royal Society of Chemistry; before 2018, it was published monthly. It won the Best New Journal 2011 award from the Association of Learned and Professional Society Publishers. The editor-in-chief is Andrew Ian Cooper (University of Liverpool). In January 2015, the journal moved to an open access publishing model. It has since become a diamond open access journal, with no charges to readers or authors. Abstracting and indexing The journal is abstracted and indexed in: *Science Citation Index Expanded *Current Contents/Physical, Chemical & Earth Sciences *Chemical Abstracts Service *Directory of Open Access Journals According to the ''Journal Citation Reports'', the journal has a 2023 impact factor of 7.6. See also * '' Chemical Communications'' * ''Chemical Societ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribonuclease A
Pancreatic ribonuclease family (, ''RNase'', ''RNase I'', ''RNase A'', ''pancreatic RNase'', ''ribonuclease I'', ''endoribonuclease I'', ''ribonucleic phosphatase'', ''alkaline ribonuclease'', ''ribonuclease'', ''gene S glycoproteins'', ''Ceratitis capitata alkaline ribonuclease'', ''SLSG glycoproteins'', ''gene S locus-specific glycoproteins'', ''S-genotype-assocd. glycoproteins'', ''ribonucleate 3'-pyrimidino-oligonucleotidohydrolase'') is a superfamily of pyrimidine-specific endonucleases found in high quantity in the pancreas of certain mammals and of some reptiles. Specifically, the enzymes are involved in endonucleolytic cleavage of 3'-phosphomononucleotides and 3'-phosphooligonucleotides ending in C-P or U-P with 2',3'-cyclic phosphate intermediates. Ribonuclease can unwind the RNA helix by complexing with single-stranded RNA; the complex arises by an extended multi-site cation-anion interaction between lysine and arginine residues of the enzyme and phosphate groups of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry of the isolated anion is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
Phosphates are the naturally occurring form of the element phosphorus. In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, phosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one proton gives the dihydrogen phosphate ion while removal of two protons gives the hydrogen phosphate ion . These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate or orthophosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Sulfate Precipitation
Ammonium sulfate precipitation is one of the most commonly used methods for large and laboratory scale protein purification and fractionation that can be used to separate proteins by altering their solubility in the presence of a high salt concentration. Properties Ammonium sulfate is an inorganic salt with a high solubility that disassociates into ammonium () and sulfate () in aqueous solutions. Ammonium sulfate is especially useful as a precipitant because it is highly soluble, stabilizes protein structure, has a relatively low density, is readily available, and is relatively inexpensive. Mechanism Ammonium sulfate, as well as other neutral salts, will stabilize proteins by preferential solvation. Proteins are usually stored in ammonium sulfate because it inhibits bacterial growth. With the addition of ammonium sulfate, proteins unfolded by denaturants can be pushed into their native conformations. This can be seen with the folding of recombinant proteins. The solubility ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Purification
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually Cell biology, cells, Tissue (biology), tissues, or whole organisms. Protein purification is vital for the specification of the function, structure, and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Ideally, to study a protein of interest, it must be separated from other components of the cell so that contaminants will not interfere in the examination of the protein of interest's structure and function. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity, and biological activity. The pure result may be termed protein isolate. Purpose The protein manufacturin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
London South Bank University
London South Bank University (LSBU) is a public university in Elephant and Castle, London. It is based in the London Borough of Southwark, near the South Bank of the River Thames, from which it takes its name. Founded in 1892 as the Borough Polytechnic Institute, it achieved university status in 1992 under the Further and Higher Education Act 1992. In September 2003, the university underwent its most recent name change to become London South Bank University (LSBU) and has since opened several new centres including the School of Health and Social Care, the Centre for Efficient and Renewable Energy in Buildings (CEREB), a new Student Centre, an Enterprise Centre, and a new media centre Elephant Studios. The university has students and 1,700 staff. In November 2016, the university was named the Entrepreneurial University of the Year at the Times Higher Education awards, Times Higher Education Awards. In the inaugural 2017 Teaching Excellence Framework, London South Bank Universi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic Effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and to be excluded by water. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar substances, which maximizes the entropy of water and minimizes the area of contact between water and nonpolar molecules. In terms of thermodynamics, the hydrophobic effect is the free energy change of water surrounding a solute. A positive free energy change of the surrounding solvent indicates hydrophobicity, whereas a negative free energy change implies hydrophilicity. The hydrophobic effect is responsible for the separation of a mixture of oil and water into its two components. It is also responsible for effects related to biology, including: cell membrane and vesicle formation, protein folding, insertion of membrane proteins into the nonpolar lipid environment and protein-small molecule associations. Hence the hydrophobic effect i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic Interaction
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. The term ''hydrophobic''—which comes from the Ancient Greek (), "having a fear of water", constructed Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon. revised and augmented through ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
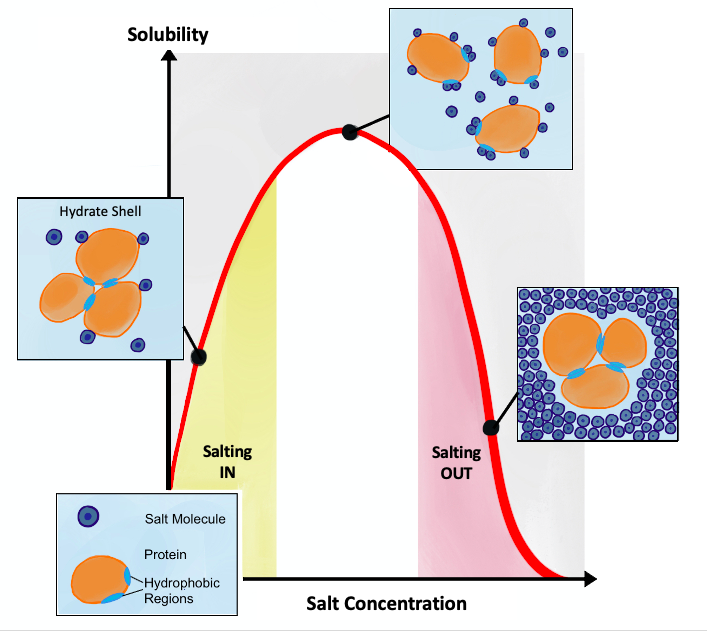
Salting Out
Salting out (also known as salt-induced precipitation, salt fractionation, anti-solvent crystallization, precipitation crystallization, or drowning out) is a purification technique that utilizes the reduced solubility of certain molecules in a solution of very high ionic strength. Salting out is typically used to precipitate large biomolecules, such as proteins or DNA. Because the salt concentration needed for a given protein to precipitate out of the solution differs from protein to protein, a specific salt concentration can be used to precipitate a target protein. This process is also used to concentrate dilute solutions of proteins. Dialysis can be used to remove the salt if needed. Principle Salt compounds dissociate in aqueous solutions. This property is exploited in the process of salting out. When the salt concentration is increased, some of the water molecules are attracted by the salt ions, which decreases the number of water molecules available to interact with the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Gerridae, water striders) to float on a water surface without becoming even partly submerged. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to Cohesion (chemistry), cohesion) than to the molecules in the air (due to adhesion). There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. This ''tangential'' force is generally referred to as the surface tension. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane. But this analogy must not be taken too far as the tension in an elastic membrane i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |