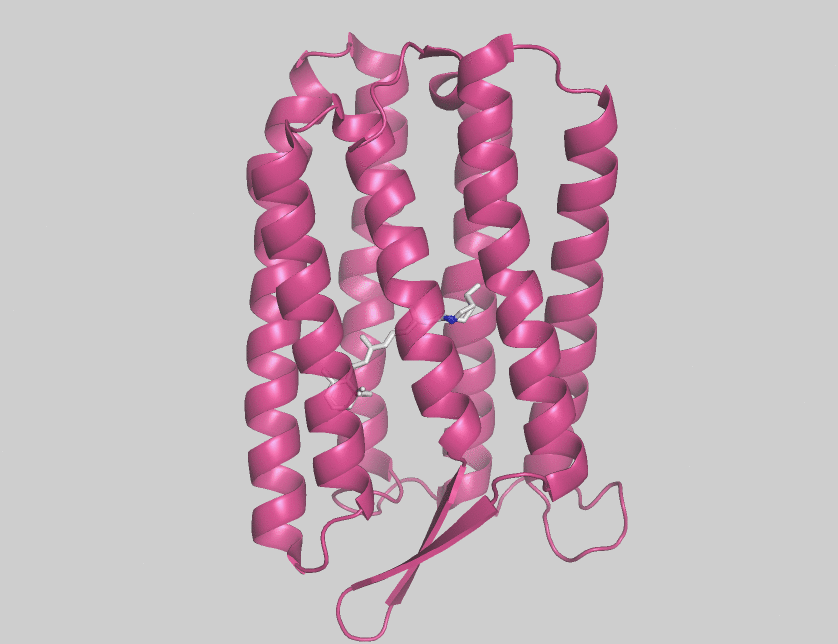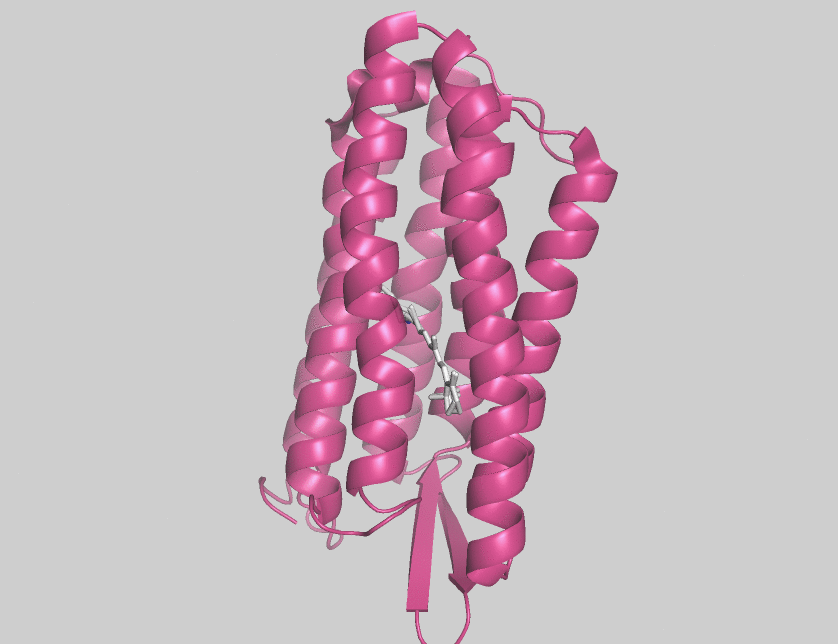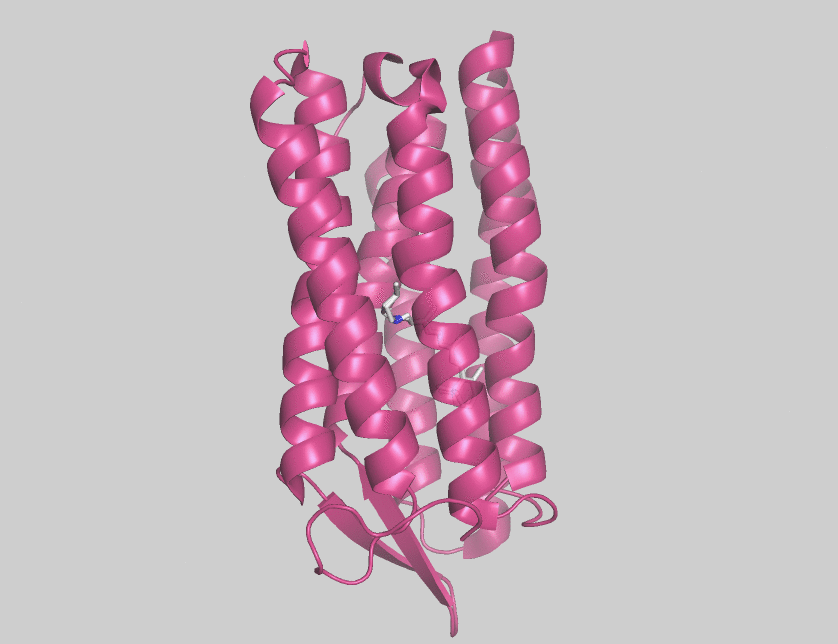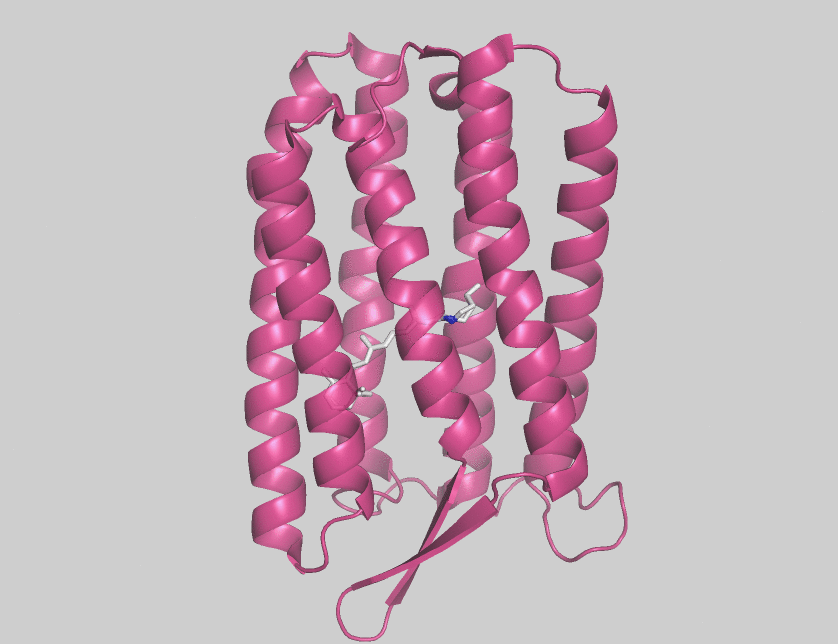|
Halorhodopsin
Halorhodopsin is a seven-transmembrane retinylidene protein from microbial rhodopsin family. It is a chloride-specific light-activated ion pump found in archaea known as halobacteria. It is activated by green light wavelengths of approximately 578 nm. Halorhodopsin also shares sequence similarity to channelrhodopsin, a light-gated ion channel. Halorhodopsin contains the essential light-isomerizable vitamin A derivative all-trans-retinal. Due to the dedication towards discovering the structure and function of this moleculc, halorhodopsin is one of the few membrane proteins whose crystal structure is known. Halorhodopsin uses the energy of green/yellow light to move chloride ions into the cell, overcoming the membrane potential. Beside chlorides it transports other halides and nitrates into the cell. Potassium chloride uptake by cells helps to maintain osmotic balance during cell growth. By performing the same task, light-driven anion pumps can considerably reduce the u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optogenetics
Optogenetics is a biological technique to control the activity of neurons or other cell types with light. This is achieved by Gene expression, expression of Channelrhodopsin, light-sensitive ion channels, Halorhodopsin, pumps or Photoactivated adenylyl cyclase, enzymes specifically in the target cells. On the level of individual Cell (biology), cells, Photoactivated adenylyl cyclase, light-activated enzymes and transcription factors allow precise control of biochemical signaling pathways. In Neuroscience, systems neuroscience, the ability to control the activity of a genetically defined set of neurons has been used to understand their contribution to decision making, learning, fear memory, mating, addiction, feeding, and locomotion. In a first medical application of optogenetic technology, vision was partially restored in a blind patient with Retinitis pigmentosa. Optogenetic techniques have also been introduced to map the Brain connectivity estimators, functional connectivity of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microbial Rhodopsin
Microbial rhodopsins, also known as bacterial rhodopsins, are retinal-binding proteins that provide light-dependent ion transport and sensory functions in halophilic and other bacteria. They are integral membrane proteins with seven transmembrane helices, the last of which contains the attachment point (a conserved lysine) for retinal. Most microbial rhodopsins pump inwards, however "mirror rhodopsins" which function outwards have been discovered. This protein family includes light-driven proton pumps, ion pumps and ion channels, as well as light sensors. For example, the proteins from halobacteria include bacteriorhodopsin and archaerhodopsin, which are light-driven proton pumps; halorhodopsin, a light-driven chloride pump; and sensory rhodopsin, which mediates both photoattractant (in the red) and photophobic (in the ultra-violet) responses. Proteins from other bacteria include proteorhodopsin. As their name indicates, microbial rhodopsins are found in Archaea and Ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optogenetics
Optogenetics is a biological technique to control the activity of neurons or other cell types with light. This is achieved by Gene expression, expression of Channelrhodopsin, light-sensitive ion channels, Halorhodopsin, pumps or Photoactivated adenylyl cyclase, enzymes specifically in the target cells. On the level of individual Cell (biology), cells, Photoactivated adenylyl cyclase, light-activated enzymes and transcription factors allow precise control of biochemical signaling pathways. In Neuroscience, systems neuroscience, the ability to control the activity of a genetically defined set of neurons has been used to understand their contribution to decision making, learning, fear memory, mating, addiction, feeding, and locomotion. In a first medical application of optogenetic technology, vision was partially restored in a blind patient with Retinitis pigmentosa. Optogenetic techniques have also been introduced to map the Brain connectivity estimators, functional connectivity of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retinylidene Protein
Retinylidene proteins, or rhodopsins in a broad sense, are proteins that use retinal as a chromophore for light reception. They are the molecular basis for a variety of light-sensing systems from phototaxis in flagellates to eyesight in animals. Retinylidene proteins include all forms of opsin and rhodopsin (in the broad sense). While rhodopsin in the narrow sense refers to a dim-light visual pigment found in vertebrates, usually on rod cells, ''rhodopsin'' in the broad sense (as used here) refers to any molecule consisting of an opsin and a retinal chromophore in the ground state. When activated by light, the chromophore is isomerized, at which point the molecule as a whole is no longer rhodopsin, but a related molecule such as metarhodopsin. However, it remains a retinylidene protein. The chromophore then separates from the opsin, at which point the bare opsin is a retinylidene protein. Thus, the molecule remains a retinylidene protein throughout the phototransduction cycle. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halobacteria
Haloarchaea (halophilic archaea, halophilic archaebacteria, halobacteria) are a class (biology), class of prokaryotic archaea under the phylum Euryarchaeota, found in water Saturated and unsaturated compounds, saturated or nearly saturated with salt. 'Halobacteria' are now recognized as archaea rather than bacteria and are one of the largest groups of archaea. The name 'halobacteria' was assigned to this group of organisms before the existence of the Domain (biology), domain Archaea was realized, and while valid according to Taxonomy (biology), taxonomic rules, should be updated. Halophilic archaea are generally referred to as haloarchaea to distinguish them from halophilic bacteria. These Halophile, halophilic microorganisms require high salt concentrations to grow, with most species requiring more than 2M NaCl for growth and survival. They are a distinct evolutionary branch of the Archaea distinguished by the possession of ether-linked lipids and the absence of murein in their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Channelrhodopsin
Channelrhodopsins are a subfamily of retinylidene proteins (rhodopsins) that function as light-gated ion channels. They serve as sensory photoreceptors in unicellular green algae, controlling phototaxis: movement in response to light. Expressed in cells of other organisms, they enable light to control electrical excitability, intracellular acidity, calcium influx, and other cellular processes (see optogenetics). Channelrhodopsin-1 (ChR1) and Channelrhodopsin-2 (ChR2) from the model organism '' Chlamydomonas reinhardtii'' are the first discovered channelrhodopsins. Variants that are sensitive to different colors of light or selective for specific ions (ACRs, KCRs) have been cloned from other species of algae and protists. History Phototaxis and photoorientation of microalgae have been studied over more than a hundred years in many laboratories worldwide. In 1980, Ken Foster developed the first consistent theory about the functionality of algal eyes. He also analyzed published a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision). Some microorganisms use retinal to convert light into metabolic energy. One study suggests that approximately three billion years ago, most living organisms on Earth used retinal, rather than chlorophyll, to convert sunlight into energy. Because retinal absorbs mostly green light and transmits purple light, this gave rise to the Purple Earth hypothesis. Retinal itself is considered to be a form of vitamin A when eaten by an animal. There are many forms of vitamin A, all of which are converted to retinal, which cannot be made without them. The number of different molecules that can be converted to retinal varies from species to species. Retinal was originally called retinene, and was renamed after it was discovered to be vitamin A aldehyde. Vertebrate animals inge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even though the domain Archaea Cladistics, cladistically includes eukaryotes, the term "archaea" (: archaeon , from the Greek "ἀρχαῖον", which means ancient) in English still generally refers specifically to prokaryotic members of Archaea. Archaea were initially Taxonomy (biology), classified as bacteria, receiving the name archaebacteria (, in the Archaebacteria Kingdom (biology), kingdom), but this term has fallen out of use. Archaeal cells have unique properties separating them from Bacteria and Eukaryote, Eukaryota. Archaea are further divided into multiple recognized phylum, phyla. Classification is difficult because most have not been Isolation (microbiology), isolated in a laboratory and have been detected only by their Gene, gene s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Channelrhodopsin-2
Channelrhodopsins are a subfamily of retinylidene proteins (rhodopsins) that function as light-gated ion channels. They serve as sensory photoreceptors in unicellular green algae, controlling phototaxis: movement in response to light. Expressed in cells of other organisms, they enable light to control electrical excitability, intracellular acidity, calcium influx, and other cellular processes (see optogenetics). Channelrhodopsin-1 (ChR1) and Channelrhodopsin-2 (ChR2) from the model organism ''Chlamydomonas reinhardtii'' are the first discovered channelrhodopsins. Variants that are sensitive to different colors of light or selective for specific ions (ACRs, KCRs) have been cloned from other species of algae and protists. History Phototaxis and photoorientation of microalgae have been studied over more than a hundred years in many laboratories worldwide. In 1980, Ken Foster developed the first consistent theory about the functionality of algal eyes. He also analyzed published act ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurons
A neuron (American English), neurone (British English), or nerve cell, is an membrane potential#Cell excitability, excitable cell (biology), cell that fires electric signals called action potentials across a neural network (biology), neural network in the nervous system. They are located in the nervous system and help to receive and conduct impulses. Neurons communicate with other cells via synapses, which are specialized connections that commonly use minute amounts of chemical neurotransmitters to pass the electric signal from the presynaptic neuron to the target cell through the synaptic gap. Neurons are the main components of nervous tissue in all Animalia, animals except sponges and placozoans. Plants and fungi do not have nerve cells. Molecular evidence suggests that the ability to generate electric signals first appeared in evolution some 700 to 800 million years ago, during the Tonian period. Predecessors of neurons were the peptidergic secretory cells. They eventually ga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperpolarization (biology)
Hyperpolarization is a change in a cell's membrane potential that makes it more negative. Cells typically have a negative resting potential, with neuronal action potentials depolarizing the membrane. When the resting membrane potential is made more negative, it increases the minimum stimulus needed to surpass the needed threshold. Neurons naturally become hyperpolarized at the end of an action potential, which is often referred to as the relative refractory period. Relative refractory periods typically last 2 milliseconds, during which a stronger stimulus is needed to trigger another action potential. Cells can also become hyperpolarized depending on channels and receptors present on the membrane, which can have an inhibitory effect. Hyperpolarization is often caused by efflux of K+ (a cation) through K+ channels, or influx of Cl– (an anion) through Cl– channels. On the other hand, influx of cations, e.g. Na+ through Na+ channels or Ca2+ through Ca2+ cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |