Microbial Rhodopsin on:
[Wikipedia]
[Google]
[Amazon]
Microbial rhodopsins, also known as bacterial rhodopsins, are retinal-binding proteins that provide light-dependent ion transport and sensory functions in
3.E.1.1.6
and others.
TC# 2.A.43
. Representative members of MR family can be found in th
Transporter Classification Database
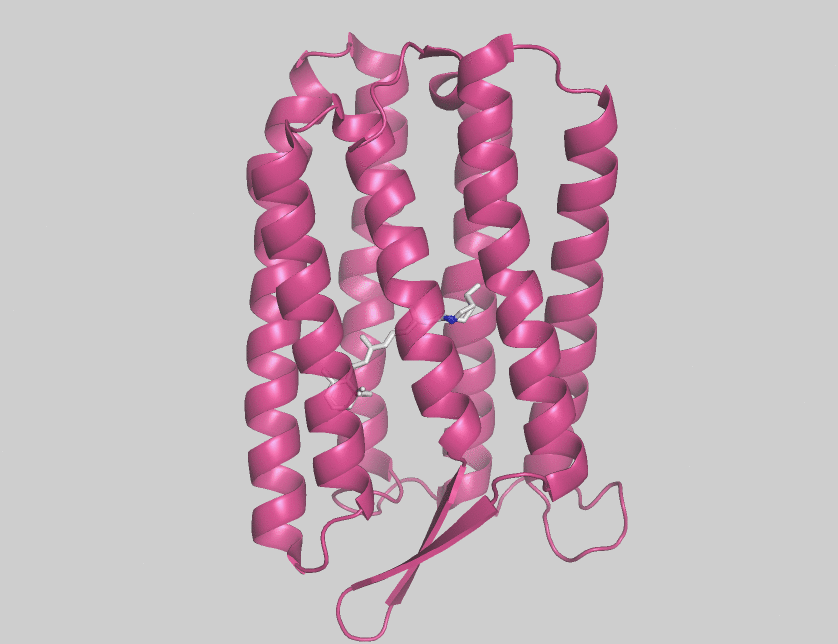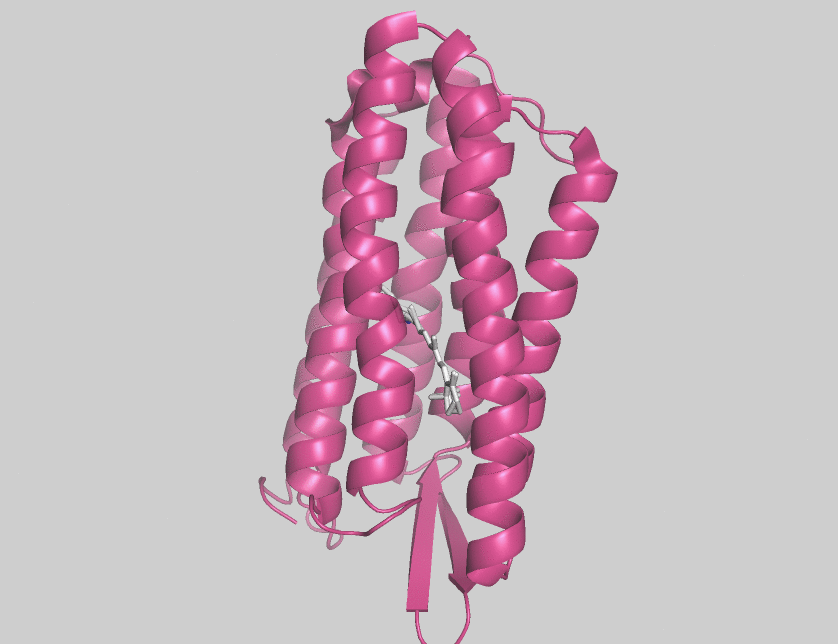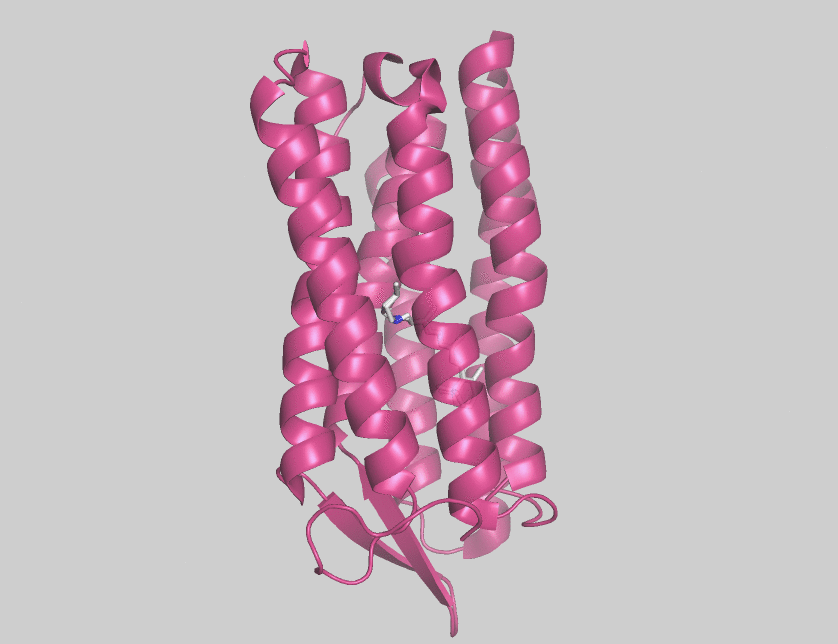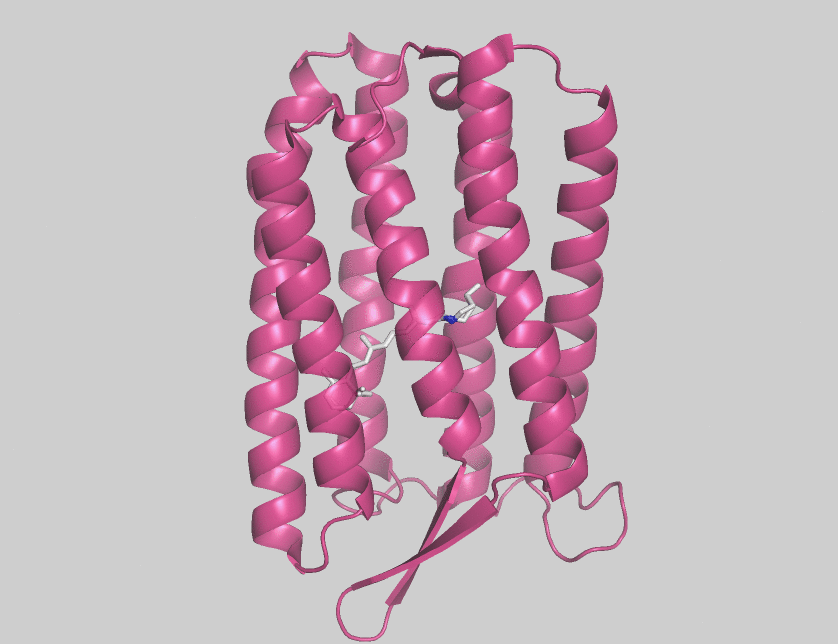 Archaerhodopsins are light-driven H+ ion transporters. They differ from bacteriorhodopsin in that the claret membrane, in which they are expressed, includes bacterioruberin, a second
Archaerhodopsins are light-driven H+ ion transporters. They differ from bacteriorhodopsin in that the claret membrane, in which they are expressed, includes bacterioruberin, a second
TC category #1.A
but because it belongs to a family in which well-characterized homologues catalyze active ion transport, it is assigned to the MR family. Expression of the ''chop1'' gene, or a truncated form of that gene encoding only the hydrophobic core (residues 1-346 or 1–517) in frog oocytes in the presence of all-trans retinal produces a light-gated conductance that shows characteristics of a channel passively but selectively permeable to protons. This channel activity probably generates bioelectric currents. A homologue of ChR1 in ''C. reinhardtii'' is channelrhodopsin-2 (ChR2; Chop2; Cop4; CSOB). This protein is 57% identical, 10% similar to ChR1. It forms a cation-selective ion channel activated by light absorption. It transports both monovalent and divalent cations. It desensitizes to a small conductance in continuous light. Recovery from desensitization is accelerated by extracellular H+ and a negative membrane potential. It may be a photoreceptor for dark adapted cells. A transient increase in hydration of transmembrane α-helices with a t(1/2) = 60 μs tallies with the onset of cation permeation. Aspartate 253 accepts the proton released by the Schiff base (t(1/2) = 10 μs), with the latter being reprotonated by aspartic acid 156 (t(1/2) = 2 ms). The internal proton acceptor and donor groups, corresponding to D212 and D115 in bacteriorhodopsin, are clearly different from other microbial rhodopsins, indicating that their spatial positions in the protein were relocated during evolution. E90 deprotonates exclusively in the nonconductive state. The observed proton transfer reactions and the protein conformational changes relate to the gating of the cation channel.
halophilic
A halophile (from the Greek word for 'salt-loving') is an extremophile that thrives in high salt concentrations. In chemical terms, halophile refers to a Lewis acidic species that has some ability to extract halides from other chemical species.
...
and other bacteria. They are integral membrane proteins with seven transmembrane helices, the last of which contains the attachment point (a conserved lysine) for retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).
Some microorganisms use ret ...
. Most microbial rhodopsins pump inwards, however "mirror rhodopsins" which function outwards have been discovered.
This protein family includes light-driven proton pumps, ion pumps and ion channels
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ...
, as well as light sensors. For example, the proteins from halobacteria
Haloarchaea (halophilic archaea, halophilic archaebacteria, halobacteria) are a class (biology), class of prokaryotic archaea under the phylum Euryarchaeota, found in water Saturated and unsaturated compounds, saturated or nearly saturated with ...
include bacteriorhodopsin and archaerhodopsin, which are light-driven proton pump
A proton pump is an integral membrane protein pump that builds up a proton gradient across a biological membrane. Proton pumps catalyze the following reaction:
: n one side of a biological membrane/sub> + energy n the other side of the m ...
s; halorhodopsin, a light-driven chloride pump; and sensory rhodopsin, which mediates both photoattractant (in the red) and photophobic (in the ultra-violet) responses. Proteins from other bacteria include proteorhodopsin.
As their name indicates, microbial rhodopsins are found in Archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even thou ...
and Bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
, and also in Eukaryota
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
(such as algae
Algae ( , ; : alga ) is an informal term for any organisms of a large and diverse group of photosynthesis, photosynthetic organisms that are not plants, and includes species from multiple distinct clades. Such organisms range from unicellular ...
) and viruses
A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea. Viruses are found in almo ...
; although they are rare in complex multicellular organism
A multicellular organism is an organism that consists of more than one cell (biology), cell, unlike unicellular organisms. All species of animals, Embryophyte, land plants and most fungi are multicellular, as are many algae, whereas a few organism ...
s.
Nomenclature
Rhodopsin was originally a synonym for " visual purple", a visualpigment
A pigment is a powder used to add or alter color or change visual appearance. Pigments are completely or nearly solubility, insoluble and reactivity (chemistry), chemically unreactive in water or another medium; in contrast, dyes are colored sub ...
(light-sensitive molecule) found in the retina
The retina (; or retinas) is the innermost, photosensitivity, light-sensitive layer of tissue (biology), tissue of the eye of most vertebrates and some Mollusca, molluscs. The optics of the eye create a focus (optics), focused two-dimensional ...
s of frogs and other vertebrate
Vertebrates () are animals with a vertebral column (backbone or spine), and a cranium, or skull. The vertebral column surrounds and protects the spinal cord, while the cranium protects the brain.
The vertebrates make up the subphylum Vertebra ...
s, used for dim-light vision, and usually found in rod cells. This is still the meaning of rhodopsin in the narrow sense, any protein evolutionarily homologous to this protein. In a broad non-genetic sense, rhodopsin refers to any molecule, whether related by genetic descent or not (mostly not), consisting of an opsin and a chromophore (generally a variant of retinal). All animal rhodopsins arose (by gene duplication and divergence) late in the history of the large G-protein coupled receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large protein family, group of evoluti ...
(GPCR) gene family, which itself arose after the divergence of plants, fungi, choanoflagellates and sponges from the earliest animals. The retinal chromophore is found solely in the opsin branch of this large gene family, meaning its occurrence elsewhere represents convergent evolution, not homology. Microbial rhodopsins are, by sequence, very different from any of the GPCR families.
The term bacterial rhodopsin originally referred to the first microbial rhodopsin discovered, known today as bacteriorhodopsin. The first bacteriorhodopsin turned out to be of archaeal origin, from ''Halobacterium salinarum
''Halobacterium salinarum'', formerly known as ''Halobacterium cutirubrum'' or ''Halobacterium halobium'', is an extremely halophile, halophilic ocean, marine obligate aerobic archaeon. Despite its name, this is not a bacteria, bacterium, but a mem ...
''. Since then, other microbial rhodopsins have been discovered, rendering the term ''bacterial rhodopsin'' ambiguous.
Table
Below is a list of some of the more well-known microbial rhodopsins and some of their properties.The ion-translocating microbial rhodopsin family
The ion-translocating microbial rhodopsin (MR) family () is a member of the TOG Superfamily of secondary carriers. Members of the MR family catalyze light-driven ion translocation across microbial cytoplasmic membranes or serve as light receptors. Most proteins of the MR family are all of about the same size (250-350 amino acyl residues) and possess seventransmembrane
A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently u ...
helical spanners with their N-termini on the outside and their C-termini on the inside. There are nine subfamilies in the MR family:
# Bacteriorhodopsins pump protons out of the cell;
# Halorhodopsins pump chloride (and other anions such as bromide, iodide and nitrate) into the cell;
# Sensory rhodopsins, which normally function as receptors for phototactic behavior, are capable of pumping protons out of the cell if dissociated from their transducer proteins;
# the Fungal Chaperones are stress-induced proteins of ill-defined biochemical function, but this subfamily also includes a H+-pumping rhodopsin;
# the bacterial rhodopsin, called Proteorhodopsin, is a light-driven proton pump that functions as does bacteriorhodopsins;
# the ''Neurospora crassa'' retinal-containing receptor serves as a photoreceptor (Neurospora ospin I);
# the green algal light-gated proton channel, Channelrhodopsin-1;
# Sensory rhodopsins from cyanobacteria.
# Light-activated rhodopsin/guanylyl cyclase
A phylogenetic analysis of microbial rhodopsins and a detailed analysis of potential examples of horizontal gene transfer
Horizontal gene transfer (HGT) or lateral gene transfer (LGT) is the movement of genetic material between organisms other than by the ("vertical") transmission of DNA from parent to offspring (reproduction). HGT is an important factor in the e ...
have been published.
Structure
Among the high resolution structures for members of the MR Family are the archaeal proteins, bacteriorhodopsin, archaerhodopsin, sensory rhodopsin II, halorhodopsin, as well as an '' Anabaena'' cyanobacterial sensory rhodopsin (TC3.E.1.1.6
and others.
Function
The association of sensory rhodopsins with their transducer proteins appears to determine whether they function as transporters or receptors. Association of a sensory rhodopsin receptor with its transducer occurs via the transmembrane helical domains of the two interacting proteins. There are two sensory rhodopsins in any one halophilic archaeon, one (SRI) that responds positively to orange light but negatively to blue light, the other (SRII) that responds only negatively to blue light. Each transducer is specific for its cognate receptor. An x-ray structure of SRII complexed with its transducer (HtrII) at 1.94 Å resolution is available (). Molecular and evolutionary aspects of the light-signal transduction by microbial sensory receptors have been reviewed.Homologues
Homologues include putative fungal chaperone proteins, a retinal-containing rhodopsin from '' Neurospora crassa'', a H+-pumping rhodopsin from ''Leptosphaeria maculans'', retinal-containing proton pumps isolated from marine bacteria, a green light-activated photoreceptor in cyanobacteria that does not pump ions and interacts with a small (14 kDa) soluble transducer protein and light-gated H+ channels from the green alga, '' Chlamydomonas reinhardtii''. The ''N. crassa'' NOP-1 protein exhibits a photocycle and conserved H+ translocation residues that suggest that this putative photoreceptor is a slow H+ pump. Most of the MR family homologues in yeast and fungi are of about the same size and topology as the archaeal proteins (283–344 amino acyl residues; seven putative transmembrane α-helical segments), but they are heat shock- and toxic solvent-induced proteins of unknown biochemical function. They have been suggested to function as pmf-driven chaperones that fold extracellular proteins, but only indirect evidence supports this postulate. The MR family is distantly related to the seven TMS LCT familyTC# 2.A.43
. Representative members of MR family can be found in th
Transporter Classification Database
Bacteriorhodopsin
Bacteriorhodopsin pumps one H+ ion, from the cytosol to the extracellular medium, per photon absorbed. Specific transport mechanisms and pathways have been proposed. The mechanism involves: # photo-isomerization of the retinal and its initial configurational changes, # deprotonation of the retinal Schiff base and the coupled release of a proton to the extracellular membrane surface, # the switch event that allows reprotonation of the Schiff base from the cytoplasmic side. Six structural models describe the transformations of the retinal and its interaction with water 402, Asp85, and Asp212 in atomic detail, as well as the displacements of functional residues farther from the Schiff base. The changes provide rationales for how relaxation of the distorted retinal causes movements of water and protein atoms that result in vectorial proton transfers to and from the Schiff base. Helix deformation is coupled to vectorial proton transport in the photocycle of bacteriorhodopsin. Most residues participating in the trimerization are not conserved in bacteriorhodopsin, a homologous protein capable of forming a trimeric structure in the absence of bacterioruberin. Despite a large alteration in the amino acid sequence, the shape of the intratrimer hydrophobic space filled by lipids is highly conserved between archaerhodopsin-2 and bacteriorhodopsin. Since a transmembrane helix facing this space undergoes a large conformational change during the proton pumping cycle, it is feasible that trimerization is an important strategy to capture special lipid components that are relevant to the protein activity.Archaerhodopsin
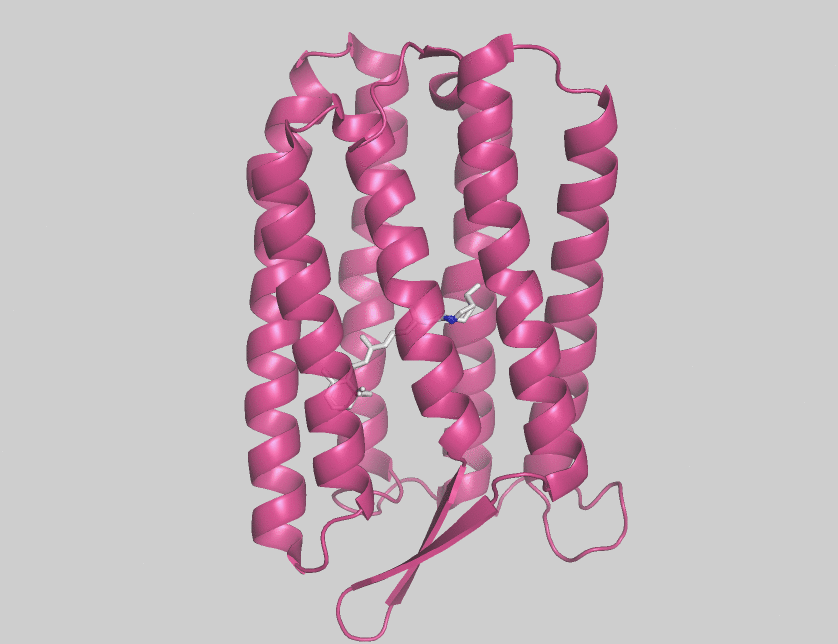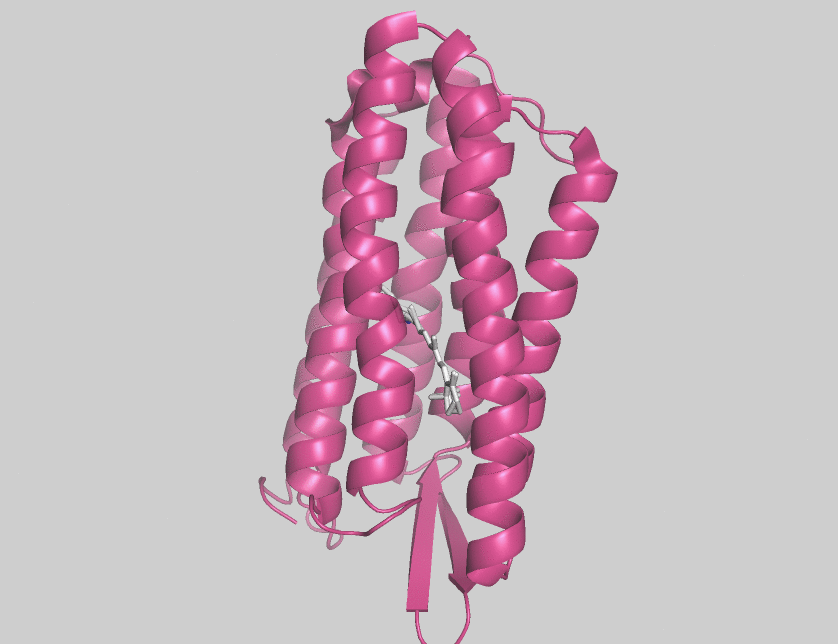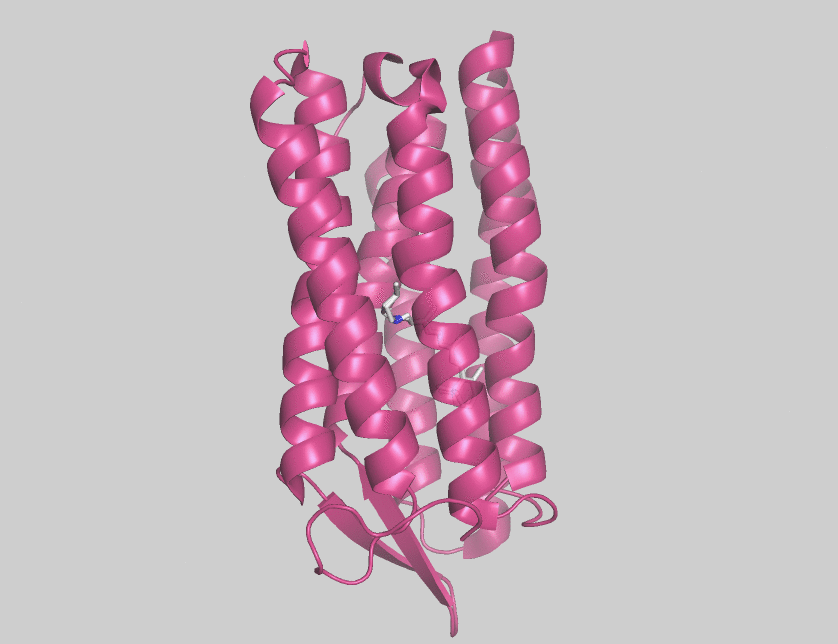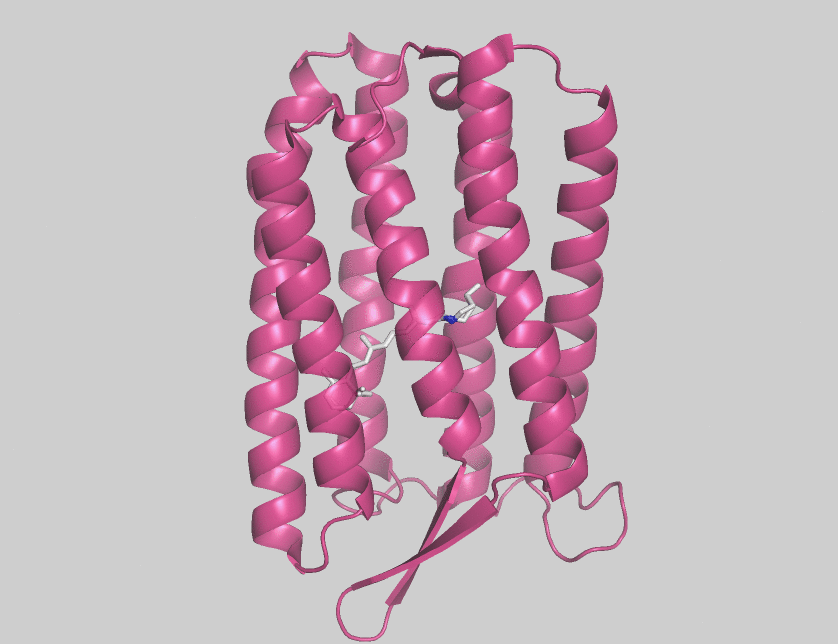 Archaerhodopsins are light-driven H+ ion transporters. They differ from bacteriorhodopsin in that the claret membrane, in which they are expressed, includes bacterioruberin, a second
Archaerhodopsins are light-driven H+ ion transporters. They differ from bacteriorhodopsin in that the claret membrane, in which they are expressed, includes bacterioruberin, a second chromophore
A chromophore is the part of a molecule responsible for its color. The word is derived .
The color that is seen by our eyes is that of the light not Absorption (electromagnetic radiation), absorbed by the reflecting object within a certain wavele ...
thought to protect against photobleaching
In optics, photobleaching (sometimes termed fading) is the photochemical alteration of a dye or a fluorophore molecule such that it is permanently unable to fluoresce. This is caused by cleaving of covalent bonds or non-specific reactions between ...
. Bacteriorhodopsin also lacks the omega loop structure that has been observed at the N-terminus of the structures of several archaerhodopsins.
Archaerhodopsin-2 (AR2) is found in the claret membrane of ''Halorubrum sp''. It is a light-driven proton pump. Trigonal and hexagonal crystals revealed that trimers are arranged on a honeycomb lattice. In these crystals, bacterioruberin binds to crevices between the subunits of the trimer. The polyene chain of the second chromophore is inclined from the membrane normal by an angle of about 20 degrees and, on the cytoplasmic side, it is surrounded by helices AB and DE of neighboring subunits. This peculiar binding mode suggests that bacterioruberin plays a structural role for the trimerization of AR2. When compared with the aR2 structure in another crystal form containing no bacterioruberin, the proton release channel takes a more closed conformation in the P321 or P6(3) crystal; i.e., the native conformation of protein is stabilized in the trimeric protein-bacterioruberin complex.
Mutants of Archaerhodopsin-3 (AR3) are widely used as tools in optogenetics
Optogenetics is a biological technique to control the activity of neurons or other cell types with light. This is achieved by Gene expression, expression of Channelrhodopsin, light-sensitive ion channels, Halorhodopsin, pumps or Photoactivated ade ...
for neuroscience research.
Channelrhodopsins
Channelrhodopsin-1 (ChR1) or channelopsin-1 (Chop1; Cop3; CSOA) of ''C. reinhardtii'' is closely related to the archaeal sensory rhodopsins. It has 712 aas with a signal peptide, followed by a short amphipathic region, and then a hydrophobic N-terminal domain with seven probable TMSs (residues 76-309) followed by a long hydrophilic C-terminal domain of about 400 residues. Part of the C-terminal hydrophilic domain is homologous to intersection (EH and SH3 domain protein 1A) of animals (AAD30271). Chop1 serves as a light-gated proton channel and mediates phototaxis and photophobic responses in green algae. Based on this phenotype, Chop1 could be assigned tTC category #1.A
but because it belongs to a family in which well-characterized homologues catalyze active ion transport, it is assigned to the MR family. Expression of the ''chop1'' gene, or a truncated form of that gene encoding only the hydrophobic core (residues 1-346 or 1–517) in frog oocytes in the presence of all-trans retinal produces a light-gated conductance that shows characteristics of a channel passively but selectively permeable to protons. This channel activity probably generates bioelectric currents. A homologue of ChR1 in ''C. reinhardtii'' is channelrhodopsin-2 (ChR2; Chop2; Cop4; CSOB). This protein is 57% identical, 10% similar to ChR1. It forms a cation-selective ion channel activated by light absorption. It transports both monovalent and divalent cations. It desensitizes to a small conductance in continuous light. Recovery from desensitization is accelerated by extracellular H+ and a negative membrane potential. It may be a photoreceptor for dark adapted cells. A transient increase in hydration of transmembrane α-helices with a t(1/2) = 60 μs tallies with the onset of cation permeation. Aspartate 253 accepts the proton released by the Schiff base (t(1/2) = 10 μs), with the latter being reprotonated by aspartic acid 156 (t(1/2) = 2 ms). The internal proton acceptor and donor groups, corresponding to D212 and D115 in bacteriorhodopsin, are clearly different from other microbial rhodopsins, indicating that their spatial positions in the protein were relocated during evolution. E90 deprotonates exclusively in the nonconductive state. The observed proton transfer reactions and the protein conformational changes relate to the gating of the cation channel.
Halorhodopsins
Bacteriorhodopsin pumps one Cl− ion, from the extracellular medium into the cytosol, per photon absorbed. Although the ions move in the opposite direction, the current generated (as defined by the movement of positive charge) is the same as for bacteriorhodopsin and the archaerhodopsins.Marine bacterial rhodopsin
A marine bacterial rhodopsin has been reported to function as a proton pump. However, it also resembles sensory rhodopsin II of archaea as well as an Orf from the fungus ''Leptosphaeria maculans'' (AF290180). These proteins exhibit 20-30% identity with each other.Transport reaction
The generalized transport reaction for bacterio- and sensory rhodopsins is: :H+ (in) + hν → H+ (out). That for halorhodopsin is: :Cl− (out) + hν → Cl− (in).See also
* Bacteriorhodopsin * Proteorhodopsin *Opsin
Animal opsins are G-protein-coupled receptors and a group of proteins made light-sensitive via a chromophore, typically retinal. When bound to retinal, opsins become retinylidene proteins, but are usually still called opsins regardless. Most pro ...
References
{{Portal bar, Biology, border=no Sensory receptors Biological pigments Protein families Membrane proteins Transmembrane proteins Transmembrane transporters Transport proteins Integral membrane proteins ru:Бактериородопсин