|
Glycerol Triglycidyl Ether
Glycerol triglycidyl ether (triglycidyl glycerol) is an aliphatic organic chemical in the glycidyl ether family. It has the formula C12H20O6. The CAS number is 13236–02–7. The IUPAC name is 2- ,3-bis(oxiran-2-ylmethoxy)propan-2-yloxymethylxirane. A key use is as a modifier for epoxy resins as a reactive diluent. Alternative names There are a variety of recognized alternate names. * Triglycidylglycerol * 1,2,3-Tris(2,3-epoxypropoxy)propane * Glycerine triglycidyl ether * Glycerol tris(2,3-epoxypropyl) ether * 2- ,3-bis(oxiran-2-ylmethoxy)propan-2-yloxymethylxirane * Propane, 1,2,3-tris(2,3-epoxypropoxy)- * Glycerol 1,2,3-triglycidyl ether Manufacture Glycerine and epichlorohydrin are reacted with a Lewis acid catalyst to form a halohydrin. The next step is dehydrochlorination with sodium hydroxide. This forms the triglycidyl ether. Uses As the molecule has 3 oxirane functionalities, it is a reactive modifier and viscosity reduction agent of epoxy resins. These reactive dilue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aliphatic Compound
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated (in which all the C-C bonds are single, requiring the structure to be completed, or 'saturated', by hydrogen) like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic. Structure Aliphatics compounds can be saturated, joined by single bonds ( alkanes), or unsaturated, with double bonds ( alkenes) or triple bonds ( alkynes). If other elements ( heteroatoms) are bound to the carbon chain, the most common being oxygen, nitrogen, sulfur, and chlorine, it is no longer a hydrocarbon, and therefore no longer an aliphatic compound. However, such compounds may still be referred to as al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly corrosive base (chemistry), base and alkali that decomposes lipids and proteins at ambient temperatures and at high concentrations may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the making of wood pulp and paper, tex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Diluents
{{disambig ...
Reactive may refer to: *Generally, capable of having a reaction (other) *An adjective abbreviation denoting a bowling ball coverstock made of reactive resin *Reactivity (chemistry) *Reactive mind *Reactive programming See also *Reactance (other) *Reactivity (other) Reactivity may refer to: * Reactivity (chemistry), the rate at which a chemical substance tends to undergo a chemical reaction * Reactive programming, a property of an execution model whereby changes are automatically propagated through a dataflo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a portmanteau of ''elastic polymer'', is often used interchangeably with ''rubber'', although the latter is preferred when referring to vulcanisates. Each of the monomers which link to form the polymer is usually a compound of several elements among carbon, hydrogen, oxygen and silicon. Elastomers are amorphous polymers maintained above their glass transition temperature, so that considerable molecular reconformation is feasible without breaking of covalent bonds. Rubber-like solids with elastic properties are called elastomers. Polymer chains are held together in these materials by relatively weak intermolecular bonds, which permit the polymers to stretch in response to macroscopic stresses. Elastomers are usually thermosets (requiring vulcanization ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
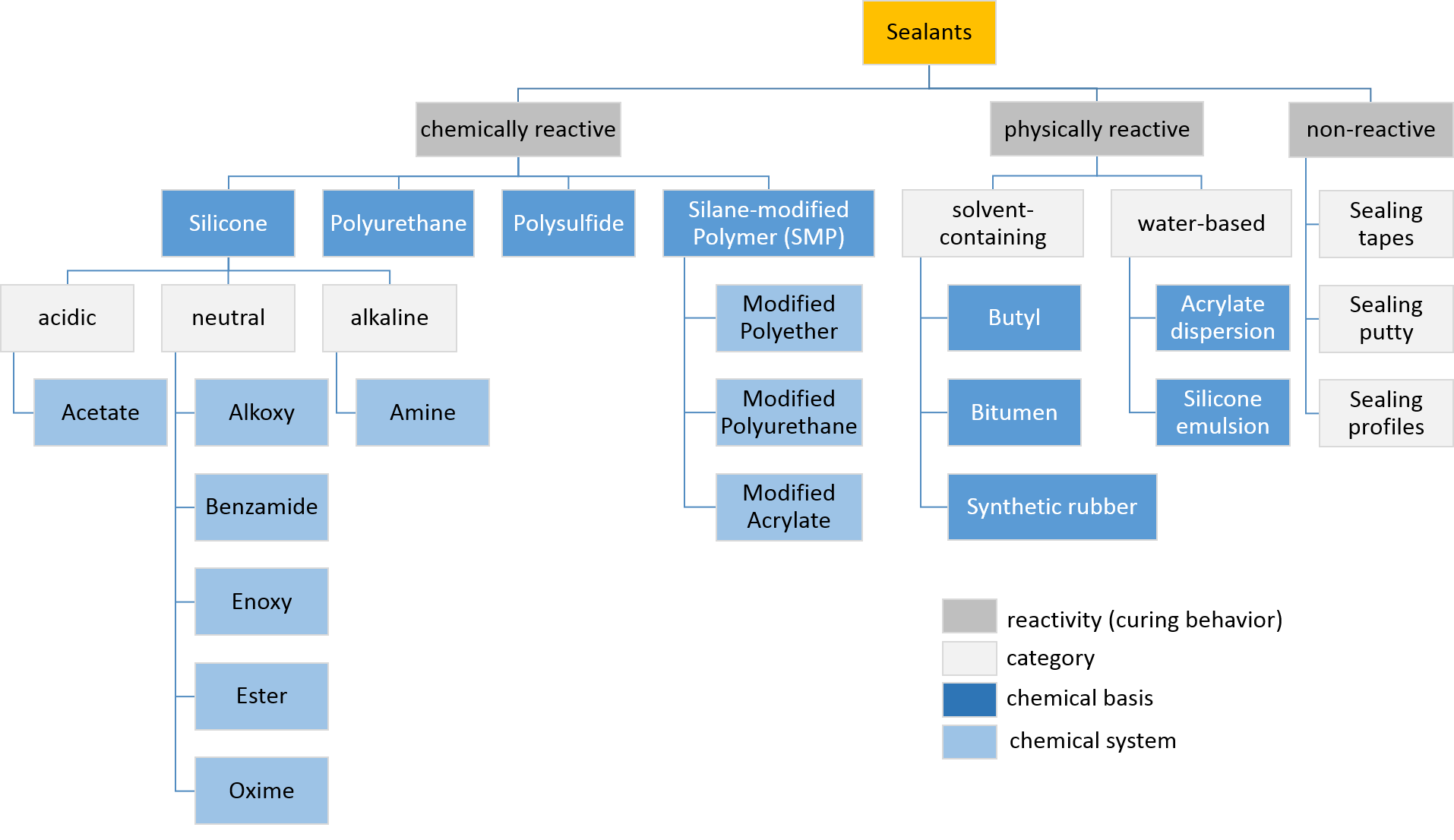
Sealant
Sealant is a substance used to block the passage of fluids through openings in materials, a type of Seal (mechanical), mechanical seal. In building construction ''sealant'' is sometimes synonymous with ''caulk'' (especially if acrylic latex or polyurethane based) and also serve the purposes of blocking dust, sound and heat transmission. Sealants may be weak or strong, flexible or rigid, permanent or temporary. Sealants are not adhesives but some have adhesive qualities and are called ''adhesive-sealants'' or ''structural sealants''. History Sealants were first used in prehistory in the broadest sense as mud, grass and reeds to seal dwellings from the weather such as the daub in wattle and daub and thatching. Natural sealants and adhesive-sealants included plant resins such as pine pitch and birch pitch, bitumen, wax, tar, natural gum, clay (mud) mortar, lime mortar, lead, blood and egg. In the 17th century glazing putty was first used to seal window glass made with linseed oil a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adhesive
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation. The use of adhesives offers certain advantages over other binding techniques such as sewing, mechanical fastenings, and welding. These include the ability to bind different materials together, the more efficient distribution of stress across a joint, the cost-effectiveness of an easily mechanized process, and greater flexibility in design. Disadvantages of adhesive use include decreased stability at high temperatures, relative weakness in bonding large objects with a small bonding surface area, and greater difficulty in separating objects during testing. Adhesives are typically organized by the method of adhesion followed by ''reactive'' or ''non-reactive'', a term which refers to whether the adhesive chemically reacts in order to harden. Alternatively, they can be organized either ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coating
A coating is a covering that is applied to the surface of an object, or substrate. The purpose of applying the coating may be decorative, functional, or both. Coatings may be applied as liquids, gases or solids e.g. powder coatings. Paints and lacquers are coatings that mostly have dual uses, which are protecting the substrate and being decorative, although some artists paints are only for decoration, and the paint on large industrial pipes is for identification (e.g. blue for process water, red for fire-fighting control) in addition to preventing corrosion. Along with corrosion resistance, functional coatings may also be applied to change the surface properties of the substrate, such as adhesion, wettability, or wear resistance.Howarth G.A "Synthesis of a legislation compliant corrosion protection coating system based on urethane, oxazolidine and waterborne epoxy technology" Master of Science Thesis April 1997 Imperial College London In other cases the coating adds a co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Oxide
Ethylene oxide is an organic compound with the chemical formula, formula . It is a cyclic ether and the simplest epoxide: a three-membered ring (chemistry), ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of a silver catalyst. The reactivity that is responsible for many of ethylene oxide's hazards also makes it useful. Although too dangerous for direct household use and generally unfamiliar to consumers, ethylene oxide is used for making many consumer products as well as non-consumer chemicals and intermediates. These products include detergents, thickeners, solvents, plastics, and various organic chemicals such as ethylen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Novartis
Novartis AG is a Swiss multinational corporation, multinational pharmaceutical company, pharmaceutical corporation based in Basel, Switzerland. Novartis is one of the largest pharmaceutical companies in the world and was the eighth largest by revenue in 2024. Novartis manufactures the drugs clozapine (Clozaril), diclofenac (Voltaren; sold to GlaxoSmithKline in 2015 deal), carbamazepine (Tegretol), valsartan (Diovan), imatinib mesylate (Gleevec/Glivec), cyclosporine (Neoral/Sandimmune), letrozole (Femara), methylphenidate (Ritalin; produced by Sandoz since 2023), terbinafine (Lamisil), deferasirox (Exjade), and others. Novartis was formed in 1996 by the merger of Ciba-Geigy and Sandoz. It was considered the largest corporate merger in history during that time. The pharmaceutical and agrochemical divisions of both companies formed Novartis as an independent entity. The name Novartis was based on the Latin terms, ''novae artes'' (new skills). After the merger, other Ciba-Geigy and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydrochlorination
In chemistry, dehydrohalogenation is an elimination reaction which removes a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications. Dehydrohalogenation from alkyl halides Traditionally, alkyl halides are substrates for dehydrohalogenations. The alkyl halide must be able to form an alkene, thus halides having no C–H bond on an adjacent carbon are not suitable substrates. Aryl halides are also unsuitable. Upon treatment with strong base, chlorobenzene dehydrohalogenates to give phenol via a benzyne intermediate. Base-promoted reactions to alkenes When treated with a strong base many alkyl chlorides convert to corresponding alkene. It is also called a β-elimination reaction and is a type of elimination reaction. Some prototypes are shown below: :\begin \ce\ &\ce \\ \ce\ &\ce \\ \ce\ &\ce \end Here ethyl chloride reacts with potassium hydroxide, typically in a solvent such as ethanol, giving ethylene. Lik ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halohydrin
In organic chemistry a halohydrin (also a haloalcohol or β-halo alcohol) is a functional group in which a halogen and a hydroxyl are bonded to adjacent carbon atoms, which otherwise bear only hydrogen or hydrocarbyl groups (e.g. 2-chloroethanol, 3-chloropropane-1,2-diol). The term only applies to saturated motifs, as such compounds like 2-chlorophenol would not normally be considered halohydrins. Megatons of some chlorohydrins, e.g. propylene chlorohydrin, are produced annually as precursors to polymers. Halohydrins may be categorized as chlorohydrins, bromohydrins, fluorohydrins or iodohydrins depending on the halogen present. Synthesis From alkenes Halohydrins are usually prepared by treatment of an alkene with a halogen, in the presence of water. The reaction is a form of electrophilic addition, with the halogen acting as electrophile. In that regard, it resembles the halogen addition reaction and proceeds with anti addition, leaving the newly added X and OH groups in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |