|
Gantacurium
Gantacurium chloride (formerly recognized as GW280430A and as AV430A) is a new experimental neuromuscular blocking drug or skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, used adjunctively in surgical anesthesia to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation. Gantacurium is not yet available for widespread clinical use: it is currently undergoing Phase III clinical development. History Gantacurium represents the third generation of tetrahydroisoquinolinium (THIQ) neuromuscular blocking drugs in a long lineage of compounds invented by medicinal chemists and scientists at Burroughs Wellcome Co., Research Triangle Park, North Carolina. Unlike all other clinically used tetrahydroisoquinolinium agents except cisatracurium, gantacurium is a stereo- and regioselective single isomer. And unlike any other traditional symmetrical predecessors in the family of ''bis''benzylte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cornell Medical Center
The Joan & Sanford I. Weill Medical College of Cornell University is Cornell University's biomedical research unit and medical school located in Upper East Side, Manhattan, New York City, New York. Weill Cornell Medicine is affiliated with NewYork-Presbyterian Hospital, Weill Cornell Medical Center, Hospital for Special Surgery, Memorial Sloan Kettering Cancer Center, and Rockefeller University, all of which are located nearby on York Avenue. Weill Cornell's clinical affiliates rank highly, with the NewYork-Presbyterian Hospital ranked #1 in the region and #4 in the nation, the Hospital for Special Surgery ranked #1 in the nation for orthopedics and the Memorial Sloan Kettering Center #2 for cancer. Memorial Sloan Kettering Cancer Center and Rockefeller University joined Weill Cornell to establish the Tri-Institutional MD–PhD Program in 1991. In 2001, the school opened a campus in Qatar. Weill Cornell has also been affiliated with Houston Methodist Hospital since 2004. On Se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BW A444
BW A444U was an experimental neuromuscular blocking drug or skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, intended to be used adjunctively in surgical anesthesia to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation. It was synthesized and developed in the early 1980s. BW A444U represented the first-generation of tetrahydroisoquinoline neuromuscular-blocking drugs that are nicotinic acetylcholine receptor antagonists or antinicotinics. It was an intermediate-duration non-depolarizing neuromuscular-blocking drug or skeletal muscle relaxant. It was synthesized by Mary M. Jackson and James C. Wisowaty, PhD (both chemists within the Chemical Development Laboratories at Burroughs Wellcome Co., Research Triangle Park, NC) in collaboration with John J. Savarese MD (who at the time was an anesthesiologist in the Dept. of Anesthesia, Harvard Medical School at the Massachusetts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mivacurium
Mivacurium chloride (formerly recognized as BW1090U81, BW B1090U or BW1090U) is a short-duration non-depolarizing neuromuscular-blocking drug or skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, used adjunctively in anesthesia to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation. Structure Mivacurium is a symmetrical molecule existing as a mixture of three of twenty possible isomers: the isomerism stems from chirality at the C-1 carbon position of both the tetrahydroisoquinolinium rings, as well as both the positively charged nitrogen (onium) heads, and the E/Z diastereomerism at the C=C double bond of the oct-4-ene diester bridge. Thus, owing to the symmetry and chirality, the three isomers of mivacurium are (E)-1R,1'R,2R,2'R, (identified as BW1217U84), (E)-1R,1'R,2R,2'S, (BW1333U83) and (E)-1R,1'R,1'S,2'S, (BW1309U83). These are also known as ''cis''-''cis'', ''cis'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Doxacurium
Doxacurium chloride (formerly recognized as BW938U80 or BW A938U) is a neuromuscular-blocking drug or skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, used adjunctively in anesthesia to provide skeletal muscle relaxation during surgery or mechanical ventilation. Unlike a number of other related skeletal muscle relaxants, it is rarely used adjunctively to facilitate endotracheal intubation. Chemistry Doxacurium is a symmetrical molecule because it is a diester of succinic acid. The pharmacological action of doxacurium is a function of its competitive antagonism to acetylcholine receptors of the nicotinic type. The drug is marketed worldwide under the tradename of Nuromax, and it is classified as a long-duration non-depolarizing neuromuscular blocking agent in a class of compounds commonly and most erroneously referred to as "benzylisoquinolines" when, in fact, it is a ''bis''benzyltetrahydroisoquinolinium agent. The pharmaceutical prepar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atracurium
Atracurium besilate, also known as atracurium besylate, is a medication used in addition to other medications to provide skeletal muscle relaxation during surgery or mechanical ventilation. It can also be used to help with endotracheal intubation but suxamethonium (succinylcholine) is generally preferred if this needs to be done quickly. It is given by injection into a vein. Effects are greatest at about 4 minutes and last for up to an hour. Common side effects include flushing of the skin and low blood pressure. Serious side effects may include allergic reactions; however, it has not been associated with malignant hyperthermia. Prolonged paralysis may occur in people with conditions like myasthenia gravis. It is unclear if use in pregnancy is safe for the baby. Atracurium is in the neuromuscular-blocker family of medications and is of the non-depolarizing type. It works by blocking the action of acetylcholine on skeletal muscles. Atracurium was approved for medical use i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glaxo Inc
GSK plc, formerly GlaxoSmithKline plc, is a British multinational pharmaceutical and biotechnology company with global headquarters in London, England. Established in 2000 by a merger of Glaxo Wellcome and SmithKline Beecham. GSK is the tenth largest pharmaceutical company and #294 on the 2022 ''Fortune'' Global 500, ranked behind other pharmaceutical companies China Resources, Sinopharm, Johnson & Johnson, Pfizer, Roche, AbbVie, Novartis, Bayer, and Merck. The company has a primary listing on the London Stock Exchange and is a constituent of the FTSE 100 Index. , it had a market capitalisation of £70 billion, the eighth largest on the London Stock Exchange. It has a secondary listing on the New York Stock Exchange. The company developed the first malaria vaccine, RTS,S, which it said in 2014 it would make available for five percent above cost. Legacy products developed at GSK include several listed in the World Health Organization's List of Essential Medicines, such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
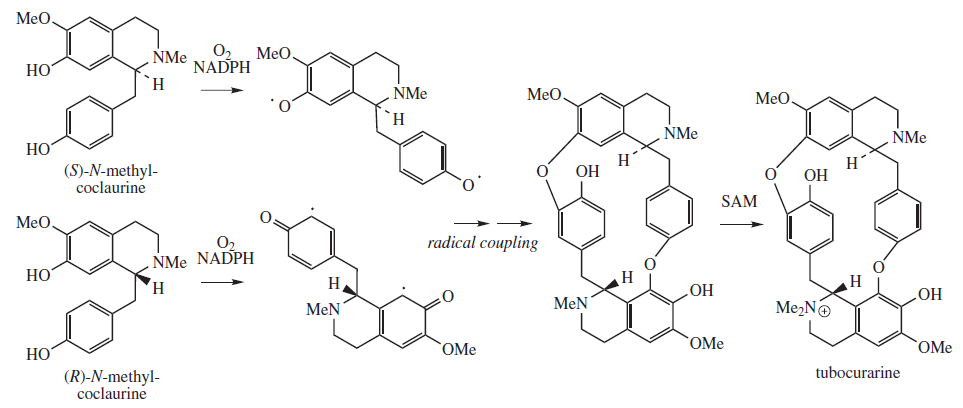
Tubocurarine
Tubocurarine (also known as ''d''-tubocurarine or DTC) is a toxic alkaloid historically known for its use as an arrow poison. In the mid-1900s, it was used in conjunction with an anesthetic to provide skeletal muscle relaxation during surgery or mechanical ventilation. It is now rarely used as an adjunct for clinical anesthesia because safer alternatives, such as cisatracurium and rocuronium, are available. History Tubocurarine is a naturally occurring mono-quaternary alkaloid obtained from the bark of the Menispermaceous South American plant '' Chondrodendron tomentosum'', a climbing vine known to the European world since the Spanish conquest of South America. Curare had been used as a source of arrow poison by South American natives to hunt animals, and they were able to eat the animals' contaminated flesh subsequently without any adverse effects because tubocurarine cannot easily cross mucous membranes. Thus, tubocurarine is effective only if given parenterally, as demonst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Suxamethonium
Suxamethonium chloride, also known as suxamethonium or succinylcholine, or simply sux by medical abbreviation, is a medication used to cause short-term paralysis as part of general anesthesia. This is done to help with tracheal intubation or electroconvulsive therapy. It is administered by injection, either into a vein or into a muscle. When used in a vein, onset of action is generally within one minute and effects last for up to 10 minutes. Common side effects include low blood pressure, increased saliva production, muscle pain, and rash. Serious side effects include malignant hyperthermia and allergic reactions. It is not recommended in people who are at risk of high blood potassium or a history of myopathy. Use during pregnancy appears to be safe for the baby. Suxamethonium is in the neuromuscular blocker family of medications and is of the depolarizing type. It works by blocking the action of acetylcholine on skeletal muscles. Side effects of succinylcholine chloride i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laudexium
Laudexium metilsulfate is a neuromuscular blocking drug or skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, used adjunctively in surgical anesthesia to facilitate endotracheal intubation and to provide skeletal muscle Skeletal muscles (commonly referred to as muscles) are organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other types of m ... relaxation during surgery or mechanical ventilation. Laudexium is no longer used in clinical practice, though it was introduced clinically in the early 1950s. It has about half the potency, a slower onset of action and a duration of action much longer than that of ''d''- tubocurarine. As with all clinically established (as well as experimental agents) with a non-depolarizing mechanism of action, its pharmacological action can be antagonized by anticholinesterases. The di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)