|
Fischer–Hafner Synthesis
114px, Structure of Cr(η6-C6H6)2 Metal arene complexes are organometallic compounds of the formula (C6R6)xMLy. Common classes are of the type (C6R6)ML3 and (C6R6)2M. These compounds are reagents in inorganic and organic synthesis. The principles that describe arene complexes extend to related organic ligands such as many heterocycles (e.g. thiophene) and polycyclic aromatic compounds (e.g. naphthalene). Synthesis Fischer–Hafner synthesis Also known as reductive Friedel–Crafts reaction, the Fischer–Hafner synthesis entails treatment of metal chlorides with arenes in the presence of aluminium trichloride and aluminium metal. The method was demonstrated in the 1950s with the synthesis of bis(benzene)chromium by Walter Hafner and his advisor E. O. Fischer. The method has been extended to other metals, e.g. u(C6Me6)2sup>2+. In this reaction, the AlCl3 serves to remove chloride from the metal precursor, and the Al metal functions as the reductant. The Fischer-Hafner synthe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ruthenium Trichloride
Ruthenium(III) chloride is the chemical compound with the formula RuCl3. "Ruthenium(III) chloride" more commonly refers to the hydrate RuCl3·''x''H2O. Both the anhydrous and hydrated species are dark brown or black solids. The hydrate, with a varying proportion of water of crystallization, often approximating to a trihydrate, is a commonly used starting material in ruthenium chemistry. Preparation and properties Anhydrous ruthenium(III) chloride is usually prepared by heating powdered ruthenium metal with chlorine. In the original synthesis, the chlorination was conducted in the presence of carbon monoxide, the product being carried by the gas stream and crystallising upon cooling. Two polymorphs of RuCl3 are known. The black α-form adopts the CrCl3-type structure with long Ru-Ru contacts of 346 pm. This polymorph has honeycomb layers of Ru3+ which are surrounded with an octahedral cage of Cl− anions. The ruthenium cations are magnetic residing in a low-spin J~1/2 ground stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Half Sandwich Compounds
One half is the multiplicative inverse of 2. It is an irreducible fraction with a numerator of 1 and a denominator of 2. It often appears in mathematical equations, recipes and measurements. As a word One half is one of the few fractions which are commonly expressed in natural languages by suppletion rather than regular derivation. In English, for example, compare the compound "one half" with other regular formations like "one-sixth". A ''half'' can also be said to be one part of something divided into two equal parts. It is acceptable to write one half as a hyphenated word, ''one-half''. Mathematics One half is the rational number that lies midway between 0 and 1 on the number line. Multiplication by one half is equivalent to division by two, or "halving"; conversely, division by one half is equivalent to multiplication by two, or "doubling". A number raised to the power of one half is equal to its square root. The area of a triangle is one half its base and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligands
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decomplexation
In chemistry, decomplexation refers to the removal of a ligand from a coordination complex. Decomplexation is of particular interest when the ligand has been synthesized within the coordination sphere of the metal, as is often the case in organometallic chemistry. Decomplexation by ligand displacement Ligands can be decomplexed by displacement with another ligand, e.g., a highly basic ligand or the use of high pressures of carbon monoxide. Arenes are liberated from (arene)Cr(CO)3 with pyridine: :(arene)Cr(CO)3 + 3 C5H5N → Cr(CO)3(NC5H5)3 + arene In this case Cr(CO)3(pyridine)3 can be recycled. Illustrative of this approach is the synthesis of (–)-steganone via a chromium haloarene complex. The synthesis is completed by decomplexation, liberating the natural product. ''(16)'' 1,4,7-Trithiacyclononane can be prepared within the coordination sphere of a metal, and then isolated by decomplexation. : Oxidative decomplexation Another popular method for decomplexation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transfer Hydrogenation
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular . It is applied in laboratory and industrial organic synthesis to saturate organic compounds and reduce ketones to alcohols, and imines to amines. It avoids the need for high-pressure molecular used in conventional hydrogenation. Transfer hydrogenation usually occurs at mild temperature and pressure conditions using organic or organometallic catalysts, many of which are chiral, allowing efficient asymmetric synthesis. It uses hydrogen donor compounds such as formic acid, isopropanol or dihydroanthracene, dehydrogenating them to , acetone, or anthracene respectively. Often, the donor molecules also function as solvents for the reaction. A large scale application of transfer hydrogenation is coal liquefaction using "donor solvents" such as tetralin. Organometallic catalysts In the area of organic synthesis, a useful family of hydrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogeneous Catalyst
In chemistry, homogeneous catalysis is catalysis where the catalyst is in same phase as reactants, principally by a soluble catalyst in a solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid and gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is an established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts. Examples Acid catalyst The proton is a pervasive homogeneous catalyst because water is the most common solvent. Water forms protons by the process of self-ionization of water. In an illustrative case, acids accelerate (catalyze) the hydrolysis of esters: :CH3CO2CH3 + H2O CH3CO2H + CH3OH At neutral pH, aqueous solutions of most esters do not hydrolyze at practical rates. Transition metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectator Ligand
In coordination chemistry, a spectator ligand is a ligand that does not participate in chemical reactions of the complex. Instead, spectator ligands (vs "actor ligands") occupy coordination sites. Spectator ligands tend to be of polydentate, such that the M-spectator ensemble is inert kinetically. Although they do not participate in reactions of the metal, spectator ligands influence the reactivity of the metal center to which they are bound. These ligands are sometimes referred to as ancillary ligands. Several different classes of ligand exist that can be considered spectator ligands. A few examples include trispyrazolylborates (Tp), cyclopentadienyl ligands (Cp), and many chelating diphosphines such as 1,2-bis(diphenylphosphino)ethane ligands (dppe). Varying the substituents on the spectator ligands greatly influences the solubility, stability, electronic, and steric properties of the metal complex. In the area of platinum-based antineoplastic Platinum-based antineoplastic drug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
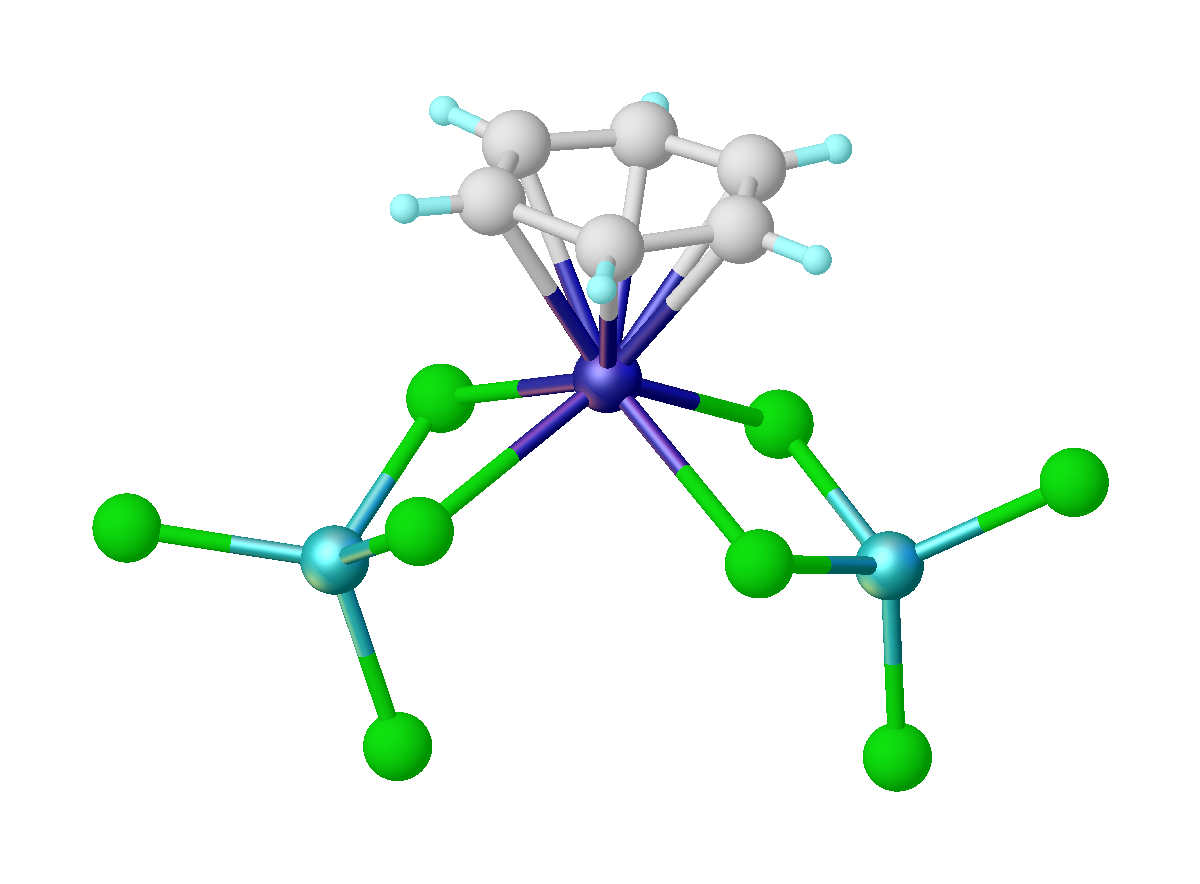
Ru(HMB)2
Ru, ru, or RU may refer to: Russia * Russia (ISO 3166-1 alpha-2 country code) * Russian language (ISO 639 alpha-2 code) * .ru, the Internet country code top-level domain for Russia China * Rù (入), the entering tone in Chinese language phonetics * Rú (儒), a Chinese language term for Confucianism * Ru (surname) (茹), a Chinese surname * Ru River (汝), in Henan, China * Ru ware, a type of Chinese pottery Educational institutions *Rajasthan University in Rajasthan, India * Radboud University Nijmegen, in Nijmegen, Netherlands * Radford University, in Virginia, USA * Rai University in Gujarat, India * Rajshahi University in Bangladesh * Rama University in India * Ramkhamhaeng University in Thailand * Rangoon University in Burma * Regis University in Colorado, USA * Reykjavík University Iceland * Rhodes University in Grahamstown, South Africa * Rockefeller University in New York, USA * Rockhurst University in Missouri, USA * Roosevelt University in Chicago, Illinois, USA * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may have hollow sphere- and ellipsoid-like forms, cylinder (geometry), tubes, or other shapes. Fullerenes with a closed mesh topology are informally denoted by their empirical formula C''n'', often written C''n'', where ''n'' is the number of carbon atoms. However, for some values of ''n'' there may be more than one isomer. The family is named after buckminsterfullerene (C60), the most famous member, which in turn is named after Buckminster Fuller. The closed fullerenes, especially C60, are also informally called buckyballs for their resemblance to the standard ball (association football), ball of association football. Nested closed fullerenes have been named bucky onions. Cylindrical fullerenes are also called carbon nanotubes or buckytubes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
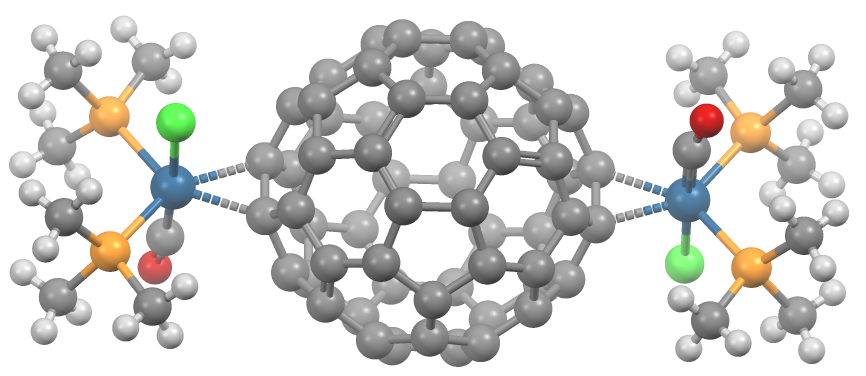
Transition Metal Fullerene Complex
A transition metal fullerene complex is a coordination complex wherein fullerene serves as a ligand. Fullerenes are typically spheroidal carbon compounds, the most prevalent being buckminsterfullerene, C60. One year after it was prepared in milligram quantities in 1990, C60 was shown to function as a ligand in the complex h3Psub>2Pt(η2-C60). Since this report, a variety of transition metals and binding modes were demonstrated. Most transition metal fullerene complex are derived from C60, although other fullerenes also coordinate to metals as seen with C70Rh(H)(CO)(PPh3)2. Binding modes As ligands, fullerenes behave similarly to electron-deficient alkenes such as tetracyanoethylene. Thus, their complexes are a subset of metal-alkene complexes. They almost always coordinate in a dihapto fashion and prefer electron-rich metal centers.Spessard, p. 162 This binding occurs on the junction of two 6-membered rings. Hexahapto and pentahapto bonding is rarely observed.Spessard, p. 165 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hapticity
In coordination chemistry, hapticity is the coordination complex, coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter eta (letter), η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated (otherwise the denticity, κ-notation is used). In addition, if the ligand coordinates through multiple atoms that are contiguous then this is considered denticity (not hapticity), and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with mu (letter), μ ('mu'), which relates to bridging ligands. History The need for additional nomenclature for organometallic compounds became apparent in the mid-1950s when Dunitz, Leslie Orgel, Orgel, and Rich described the structure of the "sandwich compound, sandwich complex" ferrocene by X-ray ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |