Fischer–Hafner Synthesis on:
[Wikipedia]
[Google]
[Amazon]
114px, Structure of Cr(η6-C6H6)2
Metal arene complexes are
organometallic compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and ...
s of the formula (C6R6)xMLy. Common classes are of the type (C6R6)ML3 and (C6R6)2M. These compounds are reagents in inorganic and organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
. The principles that describe arene complexes extend to related organic ligands such as many heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, proper ...
s (e.g. thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reacti ...
) and polycyclic aromatic compounds (e.g. naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation ...
).
Synthesis

Fischer–Hafner synthesis
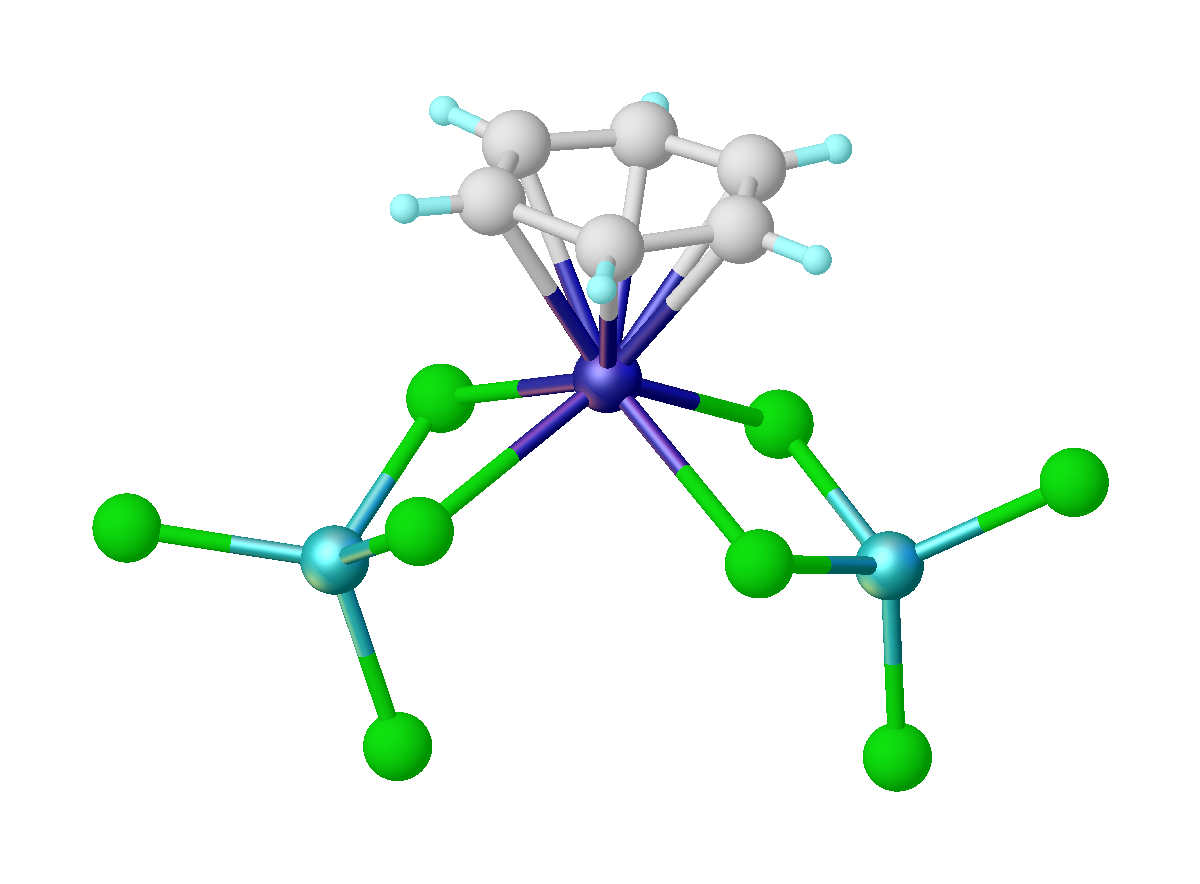
Also known as reductive Friedel–Crafts reaction, the Fischer–Hafner synthesis entails treatment of metal chlorides with arenes in the presence ofaluminium trichloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are col ...
and aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
metal. The method was demonstrated in the 1950s with the synthesis of bis(benzene)chromium
Bis(benzene)chromium is the organometallic compound with the formula . It is sometimes called dibenzenechromium. The compound played an important role in the development of sandwich compounds in organometallic chemistry and is the prototypical co ...
by Walter Hafner and his advisor E. O. Fischer. The method has been extended to other metals, e.g. u(C6Me6)2sup>2+. In this reaction, the AlCl3 serves to remove chloride from the metal precursor, and the Al metal functions as the reductant. The Fischer-Hafner synthesis is limited to arenes lacking sensitive functional groups.
114px, Structure of (Mesitylene)molybdenum tricarbonyl, Mo(η6-C6H3Me3)(CO)3.
Direct synthesis
Although many metal-arene complexes are robust, few are prepared by the direct reaction of arenes with metal salts. The main example is provided by silver perchlorate (and related salts), which dissolve in liquid arenes and crystallize with arene ligands. The strength of the metal-arene interaction is weak as indicated by the long Ag-C bond lengths and the nearly unperturbed nature of the arene. Bymetal vapor synthesis
In chemistry, metal vapor synthesis (MVS) is a method for preparing metal complexes by combining freshly produced metal atoms or small particles with ligands. In contrast to the high reactivity of such freshly produced metal atoms, bulk metals typ ...
, metal atoms co-condensed with arenes react to give complexes of the type M(arene)2. Cr(C6H6)2 can be produced by this method.
Cr(CO)6 reacts directly with benzene and other arenes to give the piano stool complex
Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples in ...
es Cr(C6R6)(CO)3. The carbonyls of Mo and W behave comparably. The method works particularly well with electron-rich arenes (e.g., anisole
Anisole, or methoxybenzene, is an organic compound with the formula . It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. The compound is mainly ...
, mesitylene
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenze ...
). The reaction has been extended to the synthesis of n(C6R6)(CO)3sup>+:
:BrMn(CO)5 + Ag+ + C6R6 → n(C6R6)(CO)3sup>+ + AgBr + 2 CO
From hexadienes
Few Ru(II) and Os(II) complexes react directly with arenes. Instead, arene complexes of these metals are typically prepared by treatment of M(III) precursors withcyclohexadiene Cyclohexadiene may refer to:
* Cyclohexa-1,3-diene,
* Cyclohexa-1,4-diene,
See also
* Benzene or its theoretical isomer ''1,3,5-Cyclohexatriene''
* Cyclohexene
{{chemistry index ...
s. For example, heating alcohol solutions of 1,3- or 1,4-cyclohexadiene and ruthenium trichloride
Ruthenium(III) chloride is the chemical compound with the formula RuCl3. "Ruthenium(III) chloride" more commonly refers to the hydrate RuCl3·''x''H2O. Both the anhydrous and hydrated species are dark brown or black solids. The hydrate, with a var ...
gives (benzene)ruthenium dichloride dimer. The conversion entails dehydrogenation of an intermediate diene complex.
Alkyne trimerization
Metal complexes are known to catalyzealkyne trimerization
An alkyne trimerisation is a +2+2nbsp; cycloaddition reaction in which three alkyne units () react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applicable to organic synthes ...
to give arenes. These reactions have been used to prepare arene complexes. Illustrative is the reaction of o(mesitylene)2sup>+ with 2-butyne to give o(C6Me6)2sup>+.
Structure
In most of its complexes, arenes bind in an η6 mode, with six nearly equidistant M-C bonds. The C-C-C angles are unperturbed vs the parent arene, but the C-C bonds are elongated by 0.2 Å. In the fullerene complex Ru3(CO)9(C60), thefullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
binds to the triangular face of the cluster.
η4- and η2-Arene complexes
In some complexes, the arene binds through only two or four carbons, η2 and η4 bonding, respectively. In these cases, the arene is no longer planar. Because the arene is dearomatized, the uncoordinated carbon centers display enhanced reactivity. A well studied example is u(η6-C6Me6)(η4-C6Me6)sup>0, formed by the reduction of u(η6-C6Me6)2sup>2+. An example of an s(η2-C6H6)(NH3)5)sup>2+.Reactivity
When bound in the η6 manner, arenes often function as unreactivespectator ligand In coordination chemistry, a spectator ligand is a ligand that does not participate in chemical reactions of the complex. Instead, spectator ligands (vs "actor ligands") occupy coordination sites. Spectator ligands tend to be of polydentate, such th ...
s, as illustrated by several homogeneous catalyst
In chemistry, homogeneous catalysis is catalysis where the catalyst is in same phase as reactants, principally by a soluble catalyst in a solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in di ...
s used for transfer hydrogenation
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular . It is applied in laboratory and industrial organic synthesis to saturate organic compounds and re ...
, such as (η6-C6R6)Ru(TsDPEN). In cationic arene complexes or those supported by several CO ligands, the arene is susceptible to attack by nucleophiles to give cyclohexadienyl derivatives.
Particularly from the perspective of organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
, the decomplexation
In chemistry, decomplexation refers to the removal of a ligand from a coordination complex. Decomplexation is of particular interest when the ligand has been synthesized within the coordination sphere of the metal, as is often the case in organome ...
of arenes is of interest. Decomplexation can often be induced by treatment with excess of ligand (MeCN, CO, etc).
References
Ligands Organometallic chemistry Coordination chemistry Transition metals {{Coordination complexes