|
Fire Clay
Fire clay is a range of refractory clays used in the manufacture of ceramics, especially fire brick. The United States Environmental Protection Agency defines fire clay very generally as a "mineral aggregate composed of hydrous silicates of aluminium (Al2O3·2SiO2·2H2O) with or without free silica." Properties High-grade fire clays can withstand temperatures of 1,775 °C (3,227 °F), but to be referred to as a "fire clay" the material must withstand a minimum temperature of .Minerals Zone, World Mineral Exchange. Retrieved 2011-6-23. Fire clays range from '' flint clays'' to ''plastic fire clays'', but there are ''semi-flint'' and ''semi-plastic'' fire clays as well. Fire clays consist of natural [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crucible
A crucible is a container in which metals or other substances may be melted or subjected to very high temperatures. Although crucibles have historically tended to be made out of clay, they can be made from any material that withstands temperatures high enough to melt or otherwise alter its contents. History Typology and chronology The form of the crucible has varied through time, with designs reflecting the process for which they are used, as well as regional variation. The earliest crucible forms derive from the sixth/fifth millennium B.C. in Eastern Europe and Iran. Chalcolithic Crucibles used for copper smelting were generally wide shallow vessels made from clay that lacks refractory properties which is similar to the types of clay used in other ceramics of the time. During the Chalcolithic period, crucibles were heated from the top by using blowpipes.Hauptmann A., 2003, ''Developments in copper Metallurgy During the Fourth and Third Millennia B.C. at Feinan'', Jordan, P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
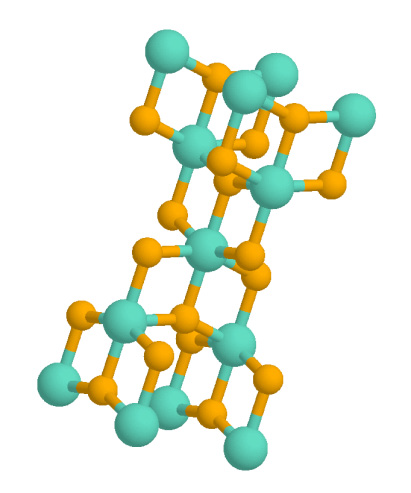
Titanium Dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound derived from titanium with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or Colour Index International, CI 77891. It is a white solid that is insoluble in water, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at a price of $13.2 billion. Structure In all three of its main dioxides, titanium exhibits Octahedral molecular geometry, octahedral geometry, being bonded to six oxide anions. The oxides in turn are bonded to three Ti centers. The overall crystal structures of rutile and anatase are tetragonal in symmetry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Oxide
Sodium oxide is a chemical compound with the formula . It is used in ceramics and glasses. It is a white solid but the compound is rarely encountered. Instead "sodium oxide" is used to describe components of various materials such as glasses and fertilizers which contain oxides that include sodium and other elements. Sodium oxide is a component. Structure The structure of sodium oxide has been determined by X-ray crystallography. Most alkali metal oxides (M = Li, Na, K, Rb) crystallise in the antifluorite structure. In this motif the positions of the anions and cations are reversed relative to their positions in , with sodium ions tetrahedrally coordinated to 4 oxide ions and oxide cubically coordinated to 8 sodium ions. Preparation Sodium oxide is produced by the reaction of sodium with sodium hydroxide, sodium peroxide, or sodium nitrite: : To the extent that NaOH is contaminated with water, correspondingly greater amounts of sodium are employed. Excess sodium is disti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Oxide
Potassium oxide ( K O) is an ionic compound of potassium and oxygen. It is a base. This pale yellow solid is the simplest oxide of potassium. It is a highly reactive compound that is rarely encountered. Some industrial materials, such as fertilizers and cements, are assayed assuming the percent composition that would be equivalent to K2O. Production Potassium oxide is produced from the reaction of oxygen and potassium; this reaction affords potassium peroxide, K2O2. Treatment of the peroxide with potassium produces the oxide: : Alternatively and more conveniently, K2O is synthesized by heating potassium nitrate with metallic potassium: : Other possibility is to heat potassium peroxide at 500 °C which decomposes at that temperature giving pure potassium oxide and oxygen. : Potassium hydroxide cannot be further dehydrated to the oxide but it can react with molten potassium to produce it, releasing hydrogen as a byproduct. : Properties and reactions K2O crystallises ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide (MgO), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from '' magnesia nigra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a mod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single compound calcium oxide. Calcium oxide that survives processing without reacting in building products, such as cement, is called free lime. Quicklime is relatively inexpensive. Both it and the chemical derivative calcium hydroxide (of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III) Oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula . It occurs in nature as the mineral hematite, which serves as the primary source of iron for the steel industry. It is also known as red iron oxide, especially when used in pigments. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (), which also occurs naturally as the mineral magnetite. Iron(III) oxide is often called rust, since rust shares several properties and has a similar composition; however, in chemistry, rust is considered an ill-defined material, described as hydrous ferric oxide. Ferric oxide is readily attacked by even weak acids. It is a weak oxidising agent, most famously when reduced by aluminium in the thermite reaction. Structure can be obtained in various polymorphs. In the primary polymorph, α, iron adopts octahedral coordination geometry. That is, each Fe center is bound to six oxygen ligands. In t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Loss On Ignition
Loss on ignition (LOI) is a test used in inorganic analytical chemistry and soil science, particularly in the analysis of minerals and the chemical makeup of soil. It consists of strongly heating ( "igniting") a sample of the material at a specified temperature, allowing volatile substances to escape, until its mass ceases to change. This may be done in air or in some other reactive or inert atmosphere. The simple test typically consists of placing a few grams of the material in a tared, pre-ignited crucible and determining its mass, placing it in a temperature-controlled furnace for a set time, cooling it in a controlled (e.g., water-free, CO2-free) atmosphere, and re-determining the mass. The process may be repeated to show that the mass change is complete. A variant of the test in which mass change is continually monitored as the temperature changes is called thermogravimetry. Theory The loss on ignition is reported as part of an elemental or oxide analysis of a mineral. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Oxide
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several Aluminium oxide (compounds), aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, ALOX or alundum in various forms and applications and alumina is refined from bauxite. It occurs naturally in its crystalline Polymorphism (materials science), polymorphic phase (matter), phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire,which have an alumina content approaching 100%. Al2O3 is used as feedstock to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point. Natural occurrence Corundum is the most common naturally occurring crystallinity, crystalline form of aluminium oxide. ruby, Rubies and sapphires are gem-quality forms o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kiln
A kiln is a thermally insulated chamber, a type of oven, that produces temperatures sufficient to complete some process, such as hardening, drying, or Chemical Changes, chemical changes. Kilns have been used for millennia to turn objects made from clay into pottery, Tile, tiles and bricks. Various industries use rotary kilns for pyroprocessing (to calcinate ores, such as limestone to Lime (material), lime for Cement kiln, cement) and to transform many other materials. Etymology According to the Oxford English Dictionary, kiln was derived from the words cyline, cylene, cyln(e) in Old English, in turn derived from Latin ''culina'' ('kitchen'). In Middle English, the word is attested as kulne, kyllne, kilne, kiln, kylle, kyll, kil, kill, keele, kiele. In Greek the word ''καίειν, kaiein'', means 'to burn'. Pronunciation The word 'kiln' was originally pronounced 'kil' with the 'n' silent, as is referenced in ''Webster's Dictionary of 1828'' and in ''English Words as Sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |