|
Endoproteinase Lys-C
Endoproteinase Lys-C is a protease that cleaves proteins on the C-terminal side of lysine residues. This enzyme is naturally found in the bacterium ''Lysobacter enzymogenes'' and is commonly used in protein sequencing. Lys-C activity is optimal in the pH range 7.0 - 9.0. See also *Trypsin *Lys-N Lys-N is a metalloendopeptidase found in the mushroom ''Grifola frondosa'' that cleaves proteins on the amino side of lysine residues. Mass spectrometry Lys-N is becoming a popular protease used for protein digestion in proteomics experiments. Th ... References Bacterial enzymes Proteases EC 3.4 Post-translational modification Proteomics §gtfgf {{Enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine prot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
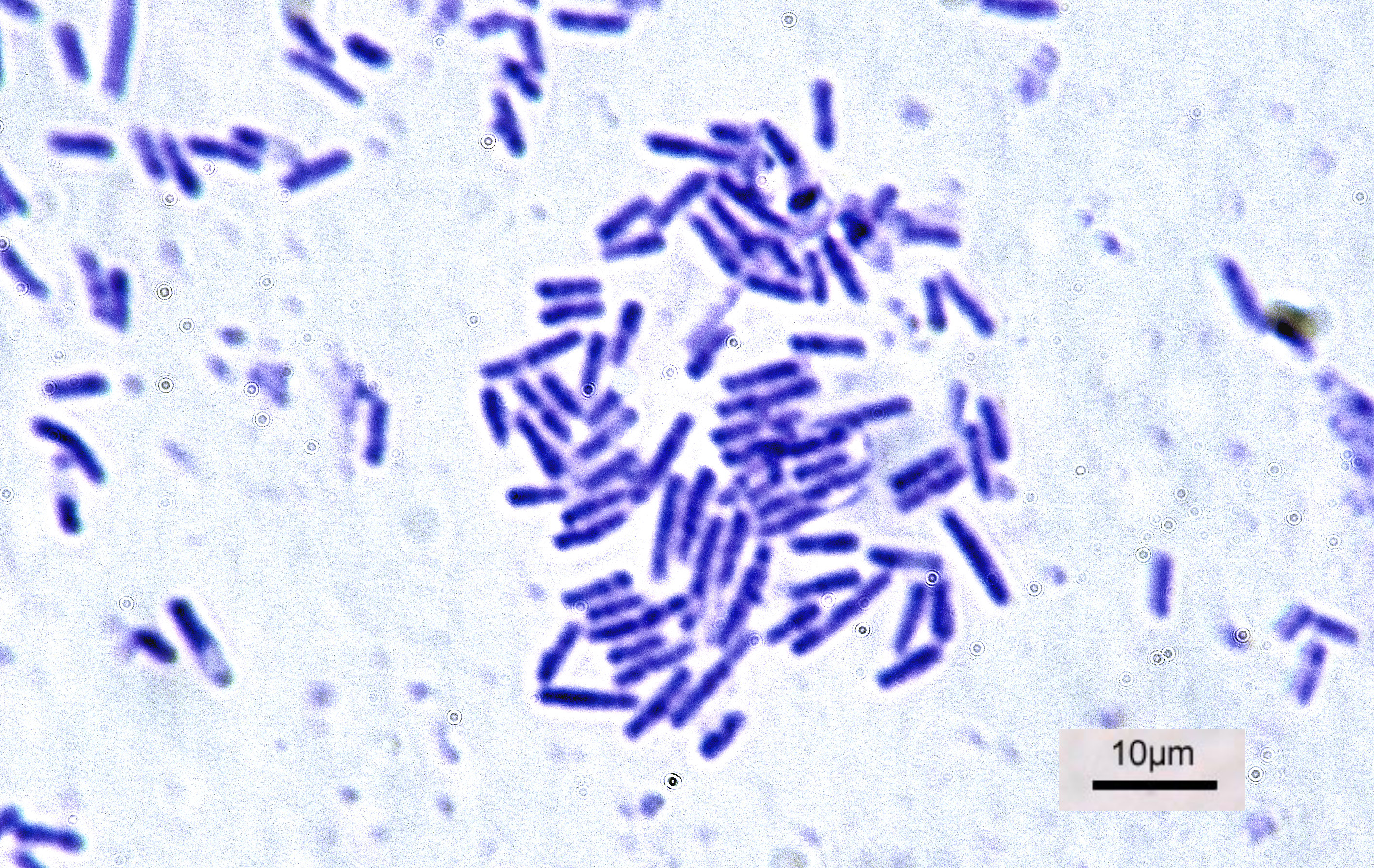
Lysobacter Enzymogenes
The genus ''Lysobacter'' belongs to the family Xanthomonadaceae within the Gammaproteobacteria and includes at least 46 named species, including: ''Lysobacter enzymogenes, L. antibioticus, L. gummosus, L. brunescens, L. defluvii, L. niabensis, L. niastensis, L. daejeonensis, L. yangpyeongensis, L. koreensis, L. concretionis, L. spongiicola'', and ''L. capsici''.Bae, H. S., W. T. Im, and S. T. Lee. 2005. ''Lysobacter concretionis'' sp. nov., isolated from anaerobic granules in an upflow anaerobic sludge blanket reactor. Int J Syst Evol Microbiol 55:1155–61.Weon, H. Y., B. Y. Kim, Y. K. Baek, S. H. Yoo, S. W. Kwon, E. Stackebrandt, and S. J. Go. 2006. Two novel species, ''Lysobacter daejeonensis'' sp. nov. and ''Lysobacter yangpyeongensis'' sp. nov., isolated from Korean greenhouse soils. Int J Syst Evol Microbiol 56:947-51. ''Lysobacter'' spp. were originally grouped with myxobacteria because they shared the distinctive trait of gliding motility, but they uniquely display a numb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Sequencing
Protein sequencing is the practical process of determining the amino acid sequence of all or part of a protein or peptide. This may serve to identify the protein or characterize its post-translational modifications. Typically, partial sequencing of a protein provides sufficient information (one or more sequence tags) to identify it with reference to databases of protein sequences derived from the conceptual translation of genes. The two major direct methods of protein sequencing are mass spectrometry and Edman degradation using a protein sequenator (sequencer). Mass spectrometry methods are now the most widely used for protein sequencing and identification but Edman degradation remains a valuable tool for characterizing a protein's ''N''-terminus. Determining amino acid composition It is often desirable to know the unordered amino acid composition of a protein prior to attempting to find the ordered sequence, as this knowledge can be used to facilitate the discovery of errors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting these long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsin proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized. Trypsin was discovered in 1876 by Wilhelm Kühne and was named from the Ancient Greek word for rubbing since it was first isolated by rubbing the pancreas with glycerin. Function In the duodenum, trypsin catalyzes the hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lys-N
Lys-N is a metalloendopeptidase found in the mushroom ''Grifola frondosa'' that cleaves proteins on the amino side of lysine residues. Mass spectrometry Lys-N is becoming a popular protease used for protein digestion in proteomics experiments. The combination Lys-N proteolytic peptides and mass spectrometry sequencing with ETD creates tandem mass spectra composed mostly of amino terminal peptide fragment ions. This fragmentation pattern facilitates the applicability of these spectra for de novo peptide sequencing. See also * Endoproteinase Lys-C * Mass spectrometry * Tandem mass spectrometry * Bottom-up proteomics References External links * The MEROPS MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was published by Rawlings & Barrett in 1993, and that for protein inhibitor ... online database for peptidases and their inhibitorsM35.004Coverage o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacterial Enzymes
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria are vital in many stages of the nutrient cycle by recycling nutrients such as the fixation of nitrogen from the atmosphere. The nutrient cycle includes the decomposition of dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, such as hydrogen sulphide and methane, to energy. Bacteria also live in symbiotic and parasitic relationships ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteases
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-translational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |