|
Dynamic Strain Aging
Dynamic strain aging (DSA) for materials science is an instability in plastic flow of materials, associated with interaction between moving dislocations and diffusing solutes. Although sometimes dynamic strain aging is used interchangeably with the Portevin–Le Chatelier effect (or serrated yielding), dynamic strain aging refers specifically to the microscopic mechanism that induces the Portevin–Le Chatelier effect. This strengthening mechanism is related to solid-solution strengthening and has been observed in a variety of fcc and bcc substitutional and interstitial alloys, metalloids like silicon, and ordered intermetallics within specific ranges of temperature and strain rate.Mesarovic, Sinisa (1995)"Dynamic Strain Aging and Plastic Instabilities." J. Mech. Phys. Solids 43:671–701 No. 5 Description of mechanism In materials, the motion of dislocations is a discontinuous process. When dislocations meet obstacles during plastic deformation (such as particles or forest di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, like in spinodal decomposition. The concept of diffusion is widely used in many fields, including physics (particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas, and price values). The central idea of diffusion, however, is common to all of these: a substance or collection undergoing diffusion spreads out from a point or location at which there is a higher concentration of that substance or collection. A gradient is the change in the value of a quantity, for example, concentration, pressure, or temperature with the change in another variable, usually distance. A change in c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatigue (material)
In materials science, fatigue is the initiation and propagation of cracks in a material due to cyclic loading. Once a fatigue crack has initiated, it grows a small amount with each loading cycle, typically producing striations on some parts of the fracture surface. The crack will continue to grow until it reaches a critical size, which occurs when the stress intensity factor of the crack exceeds the fracture toughness of the material, producing rapid propagation and typically complete fracture of the structure. Fatigue has traditionally been associated with the failure of metal components which led to the term metal fatigue. In the nineteenth century, the sudden failing of metal railway axles was thought to be caused by the metal ''crystallising'' because of the brittle appearance of the fracture surface, but this has since been disproved. Most materials, such as composites, plastics and ceramics, seem to experience some sort of fatigue-related failure. To aid in predicting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ductility
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stress before failure. Ductility is an important consideration in engineering and manufacturing. It defines a material's suitability for certain manufacturing operations (such as cold working) and its capacity to absorb mechanical overload.. Some metals that are generally described as ductile include gold and copper. However, not all metals experience ductile failure as some can be characterized with brittle failure like cast iron. Polymers generally can be viewed as ductile materials as they typically allow for plastic deformation. Malleability, a similar mechanical property, is characterized by a material's ability to deform plastically without failure under compressive stress. Historically, materials were considered malleable if they wer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
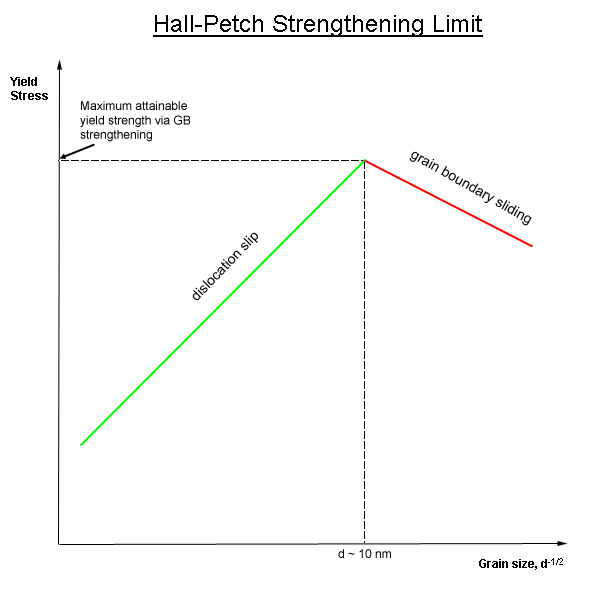
Grain Boundary Strengthening
In materials science, grain-boundary strengthening (or Hall–Petch strengthening) is a method of strengthening materials by changing their average crystallite (grain) size. It is based on the observation that grain boundaries are insurmountable borders for dislocations and that the number of dislocations within a grain has an effect on how stress builds up in the adjacent grain, which will eventually activate dislocation sources and thus enabling deformation in the neighbouring grain as well. So, by changing grain size, one can influence the number of dislocations piled up at the grain boundary and yield strength. For example, heat treatment after plastic deformation and changing the rate of solidification are ways to alter grain size.W.D. Callister. Fundamentals of Materials Science and Engineering, 2nd ed. Wiley & Sons. pp. 252. Theory In grain-boundary strengthening, the grain boundaries act as pinning points impeding further dislocation propagation. Since the lattice st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work Hardening
In materials science, work hardening, also known as strain hardening, is the strengthening of a metal or polymer by plastic deformation. Work hardening may be desirable, undesirable, or inconsequential, depending on the context. This strengthening occurs because of dislocation movements and dislocation generation within the crystal structure of the material. Many non-brittle metals with a reasonably high melting point as well as several polymers can be strengthened in this fashion. Alloys not amenable to heat treatment, including low-carbon steel, are often work-hardened. Some materials cannot be work-hardened at low temperatures, such as indium, however others can be strengthened only via work hardening, such as pure copper and aluminum. Undesirable work hardening An example of undesirable work hardening is during machining when early passes of a cutter inadvertently work-harden the workpiece surface, causing damage to the cutter during the later passes. Certain alloys are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
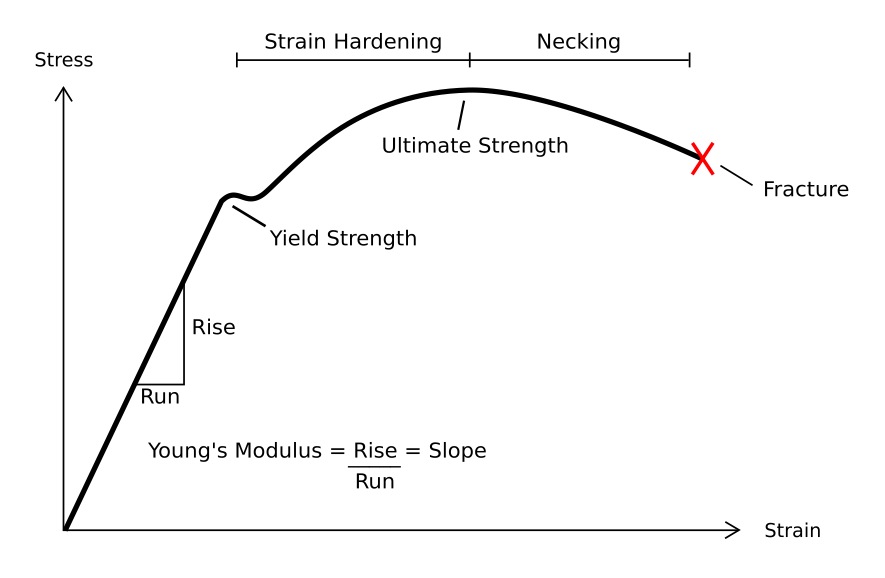
Stress–strain Curve
In engineering and materials science, a stress–strain curve for a material gives the relationship between stress (mechanics), stress and Deformation (physics)#Strain, strain. It is obtained by gradually applying Structural load, load to a test coupon and measuring the Deformation (engineering), deformation, from which the stress and strain can be determined (see tensile testing). These curves reveal many of the List of materials properties, properties of a material, such as the Young's modulus, the yield strength and the ultimate tensile strength. Definition Generally speaking, curves representing the relationship between stress and strain in any form of deformation can be regarded as stress–strain curves. The stress and strain can be normal, shear, or mixture, also can be uniaxial, biaxial, or multiaxial, even change with time. The form of deformation can be compression, stretching, torsion, rotation, and so on. If not mentioned otherwise, stress–strain curve refers to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solute Dislocation Diffusion Schematic
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Slip (materials Science)
In materials science, slip is the large displacement of one part of a crystal relative to another part along crystallographic planes and directions. Slip occurs by the passage of dislocations on close/packed planes, which are planes containing the greatest number of atoms per area and in close-packed directions (most atoms per length). Close-packed planes are known as ''slip'' or ''glide planes''. A slip system describes the set of symmetrically identical slip planes and associated family of slip directions for which dislocation motion can easily occur and lead to plastic deformation. The magnitude and direction of slip are represented by the Burgers vector, . An external force makes parts of the crystal lattice glide along each other, changing the material's geometry. A critical resolved shear stress is required to initiate a slip., Hull D., Bacon, D.J (2001); "Introduction to Dislocations", 4th ed., Slip systems Face centered cubic crystals Slip in face centered cubi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, opacity, and luster, but may have properties that differ from those of the pure metals, such as increased strength or hardness. In some cases, an alloy may reduce the overall cost of the material while preserving important properties. In other cases, the mixture imparts synergistic properties to the constituent metal elements such as corrosion resistance or mechanical strength. Alloys are defined by a metallic bonding character. The alloy constituents are usually measured by mass percentage for practical applications, and in atomic fraction for basic science studies. Alloys are usually classified as substitutional or interstitial alloys, depending on the atomic arrangement that forms the alloy. They can be further classified as homo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plastic Flow
In engineering, deformation refers to the change in size or shape of an object. ''Displacements'' are the ''absolute'' change in position of a point on the object. Deflection is the relative change in external displacements on an object. Strain is the ''relative'' internal change in shape of an infinitesimally small cube of material and can be expressed as a non-dimensional change in length or angle of distortion of the cube. Strains are related to the forces acting on the cube, which are known as stress, by a stress-strain curve. The relationship between stress and strain is generally linear and reversible up until the yield point and the deformation is elastic. The linear relationship for a material is known as Young's modulus. Above the yield point, some degree of permanent distortion remains after unloading and is termed plastic deformation. The determination of the stress and strain throughout a solid object is given by the field of strength of materials and for a structur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lüders Band
Lüders bands, is type of slip bands in metals or stretcher-strain marks which are formed due to localized bands of plastic deformation in metals experiencing tensile stresses, common to low-carbon steels and certain Al-Mg alloys. First reported by Guillaume Piobert, and later by W. Lüders, the mechanism that stimulates their appearance is known as dynamic strain aging, or the inhibition of dislocation motion by interstitial atoms (in steels, typically carbon and nitrogen), around which " atmospheres" or "zones" naturally congregate. As internal stresses tend to be highest at the shoulders of tensile test specimens, band formation is favored in those areas. However, the formation of Lüders bands depends primarily on the microscopic (i.e. average grain size and crystal structure, if applicable) and macroscopic geometries of the material. For example, a tensile-tested steel bar with a square cross-section tends to develop comparatively more bands than would a bar of identical co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |