|
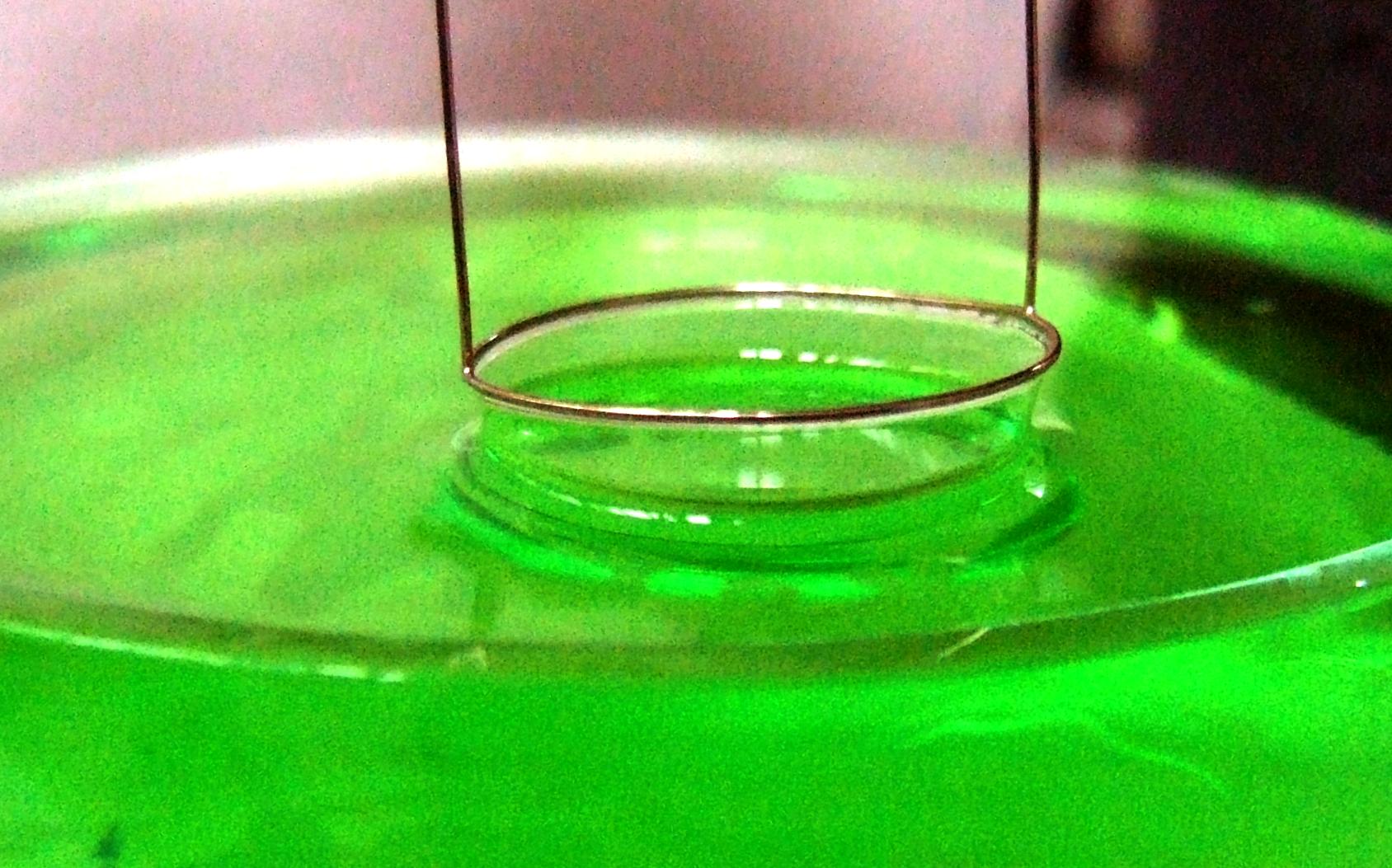
Du Noüy Ring Method
In surface science, the du Noüy ring method is a technique for measuring the surface tension of a liquid. This technique was proposed by Pierre Lecomte du Noüy in 1925. The measurement is performed with a force tensiometer, which typically uses an electrobalance to measure the excess force caused by the liquid being pulled up and automatically calculates and displays the surface tension corresponding to the force. Earlier, torsion wire balances were commonly used. Description The method involves slowly lifting a ring, often made of platinum, from the surface of a liquid. The force, , required to raise the ring from the liquid's surface is measured and related to the liquid's surface tension : : F = w_\text + 2\pi \cdot (r_\text + r_\text) \cdot \gamma, where is the radius of the inner ring of the liquid film pulled, and is the radius of the outer ring of the liquid film. is the weight of the ring minus the buoyant force due to the part of the ring below the liquid surfa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Science
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid– gas interfaces, solid– vacuum interfaces, and liquid– gas interfaces. It includes the fields of ''surface chemistry'' and '' surface physics''. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces. History The field of surface chemistry started with heterogeneous catalysis pioneered by Paul Sabatier on hydrogenation and Fritz Haber on the Haber ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Gerridae, water striders) to float on a water surface without becoming even partly submerged. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to Cohesion (chemistry), cohesion) than to the molecules in the air (due to adhesion). There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. This ''tangential'' force is generally referred to as the surface tension. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane. But this analogy must not be taken too far as the tension in an elastic membrane i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to that of a solid, and much higher than that of a gas. Therefore, liquid and solid are classified as condensed matter. Meanwhile, since both liquids and gases can flow, they are categorized as fluids. A liquid is composed of atoms or molecules held together by intermolecular bonds of intermediate strength. These forces allow the particles to move around one another while remaining closely packed. In contrast, solids have particles that are tightly bound by strong intermolecular forces, limiting their movement to small vibrations in fixed positions. Gases, on the other hand, consist of widely spaced, freely moving particles with only weak intermolecular forces. As temperature increases, the molecules in a liquid vibrate more intensely, causi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pierre Lecomte Du Noüy
Pierre Lecomte du Noüy (; 20 December 1883 – 22 September 1947) was a French biophysicist and philosopher. He is probably best remembered by scientists for his work on the surface tension, and other properties, of liquids. Early life and education Du Noüy was a descendant of the French dramatist Pierre Corneille. His mother, Hermine Lecomte du Noüy, wrote many novels, one of which, ''Amitié Amoureuse'', was translated into 16 languages and ran for 600 editions in France. Born and educated in Paris, France, du Noüy obtained the degrees of LL.B., Ph.B., Sc.B., Ph.D., and Sc.D. Career He was an associate member of the Rockefeller Institute working in Alexis Carrel's lab from 1920 through 1928, head for 10 years of the biophysics division of the Pasteur Institute, and the author of some 200 published papers. He invented a tensiometer, a scientific apparatus that used his du Noüy ring method to measure the surface tension of liquids. Du Noüy believed that mankind shou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tensiometer (surface Tension)
In surface science, a tensiometer is a measuring instrument used to measure the surface tension () of liquids or surfaces. Tensiometers are used in research and development laboratories to determine the surface tension of liquids like coatings, lacquers or adhesives. A further application field of tensiometers is the monitoring of industrial production processes like parts cleaning or electroplating. Types Goniometer/Tensiometer Surface scientists commonly use an optical goniometer/tensiometer to measure the surface tension and interfacial tension of a liquid using the pendant or sessile drop methods. A drop is produced and captured using a CCD camera. The drop profile is subsequently extracted, and sophisticated software routines then fit the theoretical Young-Laplace equation to the experimental drop profile. The surface tension can then be calculated from the fitted parameters. Unlike other methods, this technique requires only a small amount of liquid making it suitable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weighing Scale
A scale or balance is a device used to measure weight or mass. These are also known as mass scales, weight scales, mass balances, massometers, and weight balances. The traditional scale consists of two plates or bowls suspended at equal distances from a fulcrum. One plate holds an object of unknown mass (or weight), while objects of known mass or weight, called '' weights'', are added to the other plate until mechanical equilibrium is achieved and the plates level off, which happens when the masses on the two plates are equal. The perfect scale rests at neutral. A spring scale will make use of a spring of known stiffness to determine mass (or weight). Suspending a certain mass will extend the spring by a certain amount depending on the spring's stiffness (or spring constant). The heavier the object, the more the spring stretches, as described in Hooke's law. Other types of scales making use of different physical principles also exist. Some scales can be calibrate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dyne
The dyne (symbol: dyn; ) is a derived units of measurement, unit of force (physics), force specified in the centimetre–gram–second system of units, centimetre–gram–second (CGS) system of units, a predecessor of the modern International System of Units, SI. History The name dyne was first proposed as a CGS unit of force in 1873 by a Committee of the British Association for the Advancement of Science. Definition The dyne is defined as "the force required to accelerate a mass of one gram at a rate of one centimetre per second squared". An equivalent definition of the dyne is "that force which, acting for one second, will produce a change of velocity of one centimetre per second in a mass of one gram". One dyne is equal to 10 micronewtons, 10−5 newton (unit), N or to 10 nsn (nanosthenes) in the old metre–tonne–second system of units. * 1 dyn = 1 g⋅cm/s2 = 10−5 kg⋅m/s2 = 10−5 N * 1 N = 1 kg⋅m/s2 = 105 g⋅cm/s2 = 105 dyn Use The dyne per centimetr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Draper Harkins
William Draper Harkins (December 28, 1873 – March 7, 1951) was an American physical chemist, noted for his contributions to surface chemistry and nuclear chemistry. He is also recognized now as one of the first environmental chemists. Harkins researched the structure of the atomic nucleus and was the first to propose the principle of nuclear fusion, four years before Jean Baptiste Perrin published his theory in 1919-20. His findings enabled, among other things, the development of the H-bomb. As a visiting professor with Fritz Haber in 1909, he was introduced to the study of surface tension, and he began work on the theory of solutions and solubility during a visit to MIT in 1909-1910. Harkins was born in Titusville, Pennsylvania, and graduated with a PhD from Stanford University in 1907. He subsequently taught chemistry at the University of Montana from 1900 to 1912, and then spent the rest of his career at the University of Chicago. Harkins correctly predicted the existenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sessile Drop Technique
image:Contact angle.svg, 400px, An illustration of the sessile drop technique with a liquid droplet partially wetting a solid substrate. is the contact angle, and represent the solid–gas, gas–liquid, and liquid–solid interfaces, respectively. In materials science, the sessile drop technique is a method used for the characterization of solid surface energy, surface energies, and in some cases, aspects of liquid surface energies. The main premise of the method is that by placing a droplet of liquid with a known surface energy and contact angle, the surface energy of the solid substrate can be calculated. The liquid used for such experiments is referred to as the probe liquid, and the use of several different probe liquids is required. Probe liquid The surface energy is measured in units of joules per square meter, which is equivalent in the case of liquids to surface tension, measured in newtons per meter. The overall surface tension/energy of a liquid can be acquired throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilhelmy Plate
A Wilhelmy plate is a thin plate that is used to measure equilibrium surface or interfacial tension at an air–liquid or liquid–liquid interface. In this method, the plate is oriented perpendicular to the interface, and the force exerted on it is measured. Based on the work of Ludwig Wilhelmy, this method finds wide use in the preparation and monitoring of Langmuir films. Detailed description The Wilhelmy plate consists of a thin plate usually on the order of a few square centimeters in area. The plate is often made from filter paper, glass or platinum which may be roughened to ensure complete wetting. In fact, the results of the experiment do not depend on the material used, as long as the material is wetted by the liquid. The plate is cleaned thoroughly and attached to a balance with a thin metal wire. The force on the plate due to wetting is measured using a tensiometer or microbalance and used to calculate the surface tension (\gamma) using the Wilhelmy equation: :\ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |