|
Diselenide
Diselenide may refer to: * Diselane, H-Se-Se-H * Carbon diselenide, CSe2, a yellow-orange oily liquid with pungent odor * Any organic chemical compound with a selenium-selenium bond, R-Se-Se-R (see Organoselenium chemistry) ** Diphenyl diselenide, (C6H5)–Se–Se–(C6H5) ** selenocystine * Metal dichalcogenides ** Manganese diselenide (MnSe2) ** Molybdenum diselenide (MoSe2) ** Tungsten diselenide (WSe2) ** Titanium diselenide (TiSe2) {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Diselenide
Carbon diselenide is an inorganic compound with the chemical formula . It is a yellow-orange oily liquid with pungent odor. It is the selenium analogue of carbon disulfide () and carbon dioxide (). This light-sensitive compound is insoluble in water and soluble in organic solvents. Synthesis, structure and reactions Carbon diselenide is a linear molecule with D∞h molecular symmetry, symmetry. It is produced by reacting selenium powder with dichloromethane vapor near 550 °C. : It was first reported by Grimm and Metzger, who prepared it by treating hydrogen selenide with carbon tetrachloride in a hot tube. Like carbon disulfide, carbon diselenide polymerizes under high pressure. The structure of the polymer is thought to be a head-to-head structure with a backbone chain, backbone in the form of . The polymer is a semiconductor with a room-temperature conductivity of 50 S/cm. In addition, carbon diselenide is a precursor to tetraselenafulvalenes, the selenium analogue of tet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphenyl Diselenide
Diphenyl diselenide is the chemical compound with the formula (C6H5)2Se2, abbreviated Ph2Se2. This yellow-coloured solid is the oxidized derivative of benzeneselenol. It is used as a source of the PhSe unit in organic synthesis. Preparation and properties Ph2Se2 is prepared by the oxidation of benzeneselenoate, the conjugate base of benzeneselenol which is generated via the Grignard reagent: : PhMgBr + Se → PhSeMgBr :2 PhSeMgBr + Br2 → Ph2Se2 + 2 MgBr2 The molecule has idealized C2-symmetry, like hydrogen peroxide and related molecules. The Se-Se bond length of 2.29 Å the C-Se-Se-C dihedral angle is 82° and the C-Se-Se angles are near 110°. Medical applications Diphenyl diselenide alleviates methylmercury poisoning in grass carp. Reactions A reaction characteristic of Ph2Se2 is its reduction: :Ph2Se2 + 2 Na → 2 PhSeNa PhSeNa is a useful nucleophile used to introduce the phenylselenyl group by nucleophilic substitution of alkyl halides, alkyl sulfonates ( mesyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
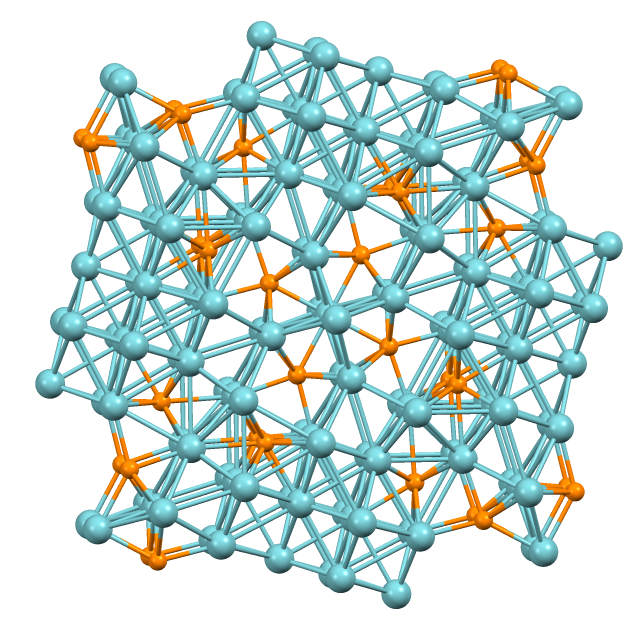
Molybdenum Diselenide
Molybdenum diselenide () is an inorganic compound of molybdenum and selenium. Its structure is similar to that of . Compounds of this category are known as transition metal dichalcogenides, abbreviated TMDCs. These compounds, as the name suggests, are made up of a transition metals and elements of group 16 on the periodic table of the elements. Compared to , exhibits higher electrical conductivity. Structure Like many TMDCs, is a layered material with strong in-plane bonding and weak out-of-plane interactions. These interactions lead to exfoliation into two-dimensional layers of single unit cell thickness. The most common form of these TMDCs have trilayers of molybdenum sandwiched between selenium ions causing a trigonal prismatic metal bonding coordination, but it is octahedral when the compound is exfoliated. The metal ion in these compounds is surrounded by six ions. The coordination geometry of the Mo is sometimes found as octahedral and trigonal prismatic.Parilla, P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diselane
Hydrogen diselenide is an inorganic selenium compound with a chemical formula or . At room temperature, hydrogen diselenide dissociates easily to hydrogen selenide () and elemental selenium Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elem ..., and is therefore not stable. However, hydrogen diselenide can be stable in some solutions. References {{Selenides Selenium compounds Hydrogen compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoselenium Chemistry
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments. Selenium can exist with oxidation state −2, +2, +4, +6. Se(II) is the dominant form in organoselenium chemistry. Down the group 16 column, the bond strength becomes increasingly weaker (234 kJ/ mol for the bond and 272 kJ/mol for the bond) and the bond lengths longer ( 198 pm, 181 pm and 141 pm). Selenium compounds are more nucleophilic than the corresponding sulfur compounds and also more acidic. The p''K''a values of are 16 for oxygen, 7 for sulfur and 3.8 for selenium. In contrast to sulfoxides, the corresponding selenoxides are unstable in the presence of β-protons and this propert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten Diselenide
Tungsten diselenide is an inorganic compound with the formula WSe2. The compound adopts a hexagonal crystalline structure similar to molybdenum disulfide. The tungsten atoms are covalently bonded to six selenium ligands in a trigonal prismatic coordination sphere while each selenium is bonded to three tungsten atoms in a pyramidal geometry. The tungsten–selenium bond has a length of 0.2526 nm, and the distance between selenium atoms is 0.334 nm. It is a well studied example of a layered material. The layers stack together via Van der Waals force, van der Waals interactions. WSe2 is a very stable semiconductor in the group-VI transition metal dichalcogenides. Structure and properties The hexagonal (P63/mmc) polymorph 2H-WSe2 is isotypic with hexagonal Molybdenum disulfide, MoS2. The two-dimensional lattice structure has W and Se arranged periodically in layers with hexagonal symmetry. Similar to graphite, van der Waals interactions hold the layers together; however, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcogenide
: 220px, Cadmium sulfide, a prototypical metal chalcogenide, is used as a yellow pigment. A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant. Alkali metal and alkaline earth chalcogenides Alkali metal and alkaline earth monochalcogenides are salt-like, being colourless and often water-soluble. The sulfides tend to undergo hydrolysis to form derivatives containing bisulfide (SH−) anions. The alkali metal chalcogenides often crystallize with the antifluorite structure and the alkal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Diselenide
Manganese diselenide is the inorganic compound with the formula MnSe2. This rarely encountered solid is structurally similar to that of iron pyrite (FeS2). Analogous to the description of iron pyrite, manganese diselenide is sometimes viewed as being composed of Mn2+ and Se22− ions, although being a semiconductor, MnSe2 is not appropriately described in formal oxidation states. Spectroscopy The high‐resolution Mn 2p spectra of the MnSe2 has two distinct peaks at 642.2 and 653.9 electronvolt In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an Voltage, electric potential difference of one volt in vacuum ...s correspond to the Mn 2p3/2 and Mn 2p1/2 spin–orbit components, respectively. The energy difference (Δ 2p) of 11.7 eV confirms the presence of Mn4+ ions in the sample. A good correlation was observed with the literature va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenocystine
Selenocystine is the amino acid with the formula . It is the oxidized derivative of the canonical amino acid selenocysteine (). The compound can also be prepared synthetically from serine. Because selenocysteine is not easily isolated or handled, it is often generated by reduction of selenocystine in situ. The selenium–selenium bond length is 2.321 Å, which is 14% longer than the disulfide bond in cystine Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine serves two functions: a site of redox reactions and a mec ... at 2.040 Å. References {{Amino acids Alpha-Amino acids Proteinogenic amino acids Organoselenium compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |