|
Diphenyl Diselenide
Diphenyl diselenide is the chemical compound with the formula (C6H5)2Se2, abbreviated Ph2Se2. This yellow-coloured solid is the oxidized derivative of benzeneselenol. It is used as a source of the PhSe unit in organic synthesis. Preparation and properties Ph2Se2 is prepared by the oxidation of benzeneselenoate, the conjugate base of benzeneselenol which is generated via the Grignard reagent: : PhMgBr + Se → PhSeMgBr :2 PhSeMgBr + Br2 → Ph2Se2 + 2 MgBr2 The molecule has idealized C2-symmetry, like hydrogen peroxide and related molecules. The Se-Se bond length of 2.29 Å the C-Se-Se-C dihedral angle is 82° and the C-Se-Se angles are near 110°. Medical applications Diphenyl diselenide alleviates methylmercury poisoning in grass carp. Reactions A reaction characteristic of Ph2Se2 is its reduction: :Ph2Se2 + 2 Na → 2 PhSeNa PhSeNa is a useful nucleophile used to introduce the phenylselenyl group by nucleophilic substitution of alkyl halides, alkyl sulfonates ( mesyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichloromethane
Dichloromethane (DCM, methylene chloride, or methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.Rossberg, M. ''et al.'' (2006) "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. . Occurrence Natural sources of dichloromethane include oceanic sources, macroalgae, wetlands, and volcanoes. However, the majority of dichloromethane in the environment is the result of industrial emissions. Production DCM is produced by treating either chloromethane or methane with chlorine gas at 400–500 °C. At these temperatures, both methane and chloromethane undergo a series of reactions producing progressively more chlorinated products. In this way, an estimated 400,000 tons were produced in the US, Europe, and Japan in 199 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group with the chemical formula . It consists of a tolyl group, , joined to a sulfonyl group, , with the open valence on sulfur. This group is usually derived from the compound tosyl chloride, (abbreviated TsCl), which forms esters and amides of toluenesulfonic acid, (abbreviated TsOH). The para orientation illustrated (''p''-toluenesulfonyl) is most common, and by convention ''tosyl'' without a prefix refers to the ''p''-toluenesulfonyl group. The tosyl terminology was proposed by German chemists Kurt Hess and Robert Pfleger in 1933 on the pattern of trityl and adopted in English starting from 1934. The toluenesulfonate (or tosylate) group refers to the (–OTs) group, with an additional oxygen attached to sulfur and open valence on an oxygen. In a chemical name, the term ''tosylate'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels–Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic chemistry has a strong tradition of naming a specif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Compound
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes, ketones and carboxylic acid), as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, such that carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides, chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The American Chemical Society
The ''Journal of the American Chemical Society'' (also known as JACS) is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical and Applied Chemistry'' (July 1893) and the ''American Chemical Journal'' (January 1914). It covers all fields of chemistry. Since 2021, the editor-in-chief is Erick M. Carreira (ETH Zurich). In 2014, the journal moved to a hybrid open access publishing model. Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2023 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a type of journal ranking. Journals with higher impact factor values are considered more prestigious or important within their field. The Impact Factor of a journa ... of 14.4. Editors-in-chief The following people are or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactam
A lactam is a Cyclic compound, cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words ''lactone'' + ''amide''. Nomenclature Greek_alphabet#Letters, Greek prefixes in alphabetical order indicate ring size. This ring-size nomenclature stems from the fact that hydrolysis of an α-lactam gives an α-amino acid and that of a β-Lactam gives a β-amino acid, and so on. Synthesis General synthetic methods are used for the organic synthesis of lactams. Beckmann rearrangement Lactams form by the Acid catalysis, acid-catalyzed Rearrangement reaction, rearrangement of oximes in the Beckmann rearrangement. Schmidt reaction Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction. Cyclohexanone with hydrazoic acid, forms Caprolactam, ε - Caprolactum, which upon treatment with excess acid forms Pentylenetetrazol, Cardiazole, a heart stimulant. Cyclization of amino acids Lactams can be formed fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organolithium Reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. History and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
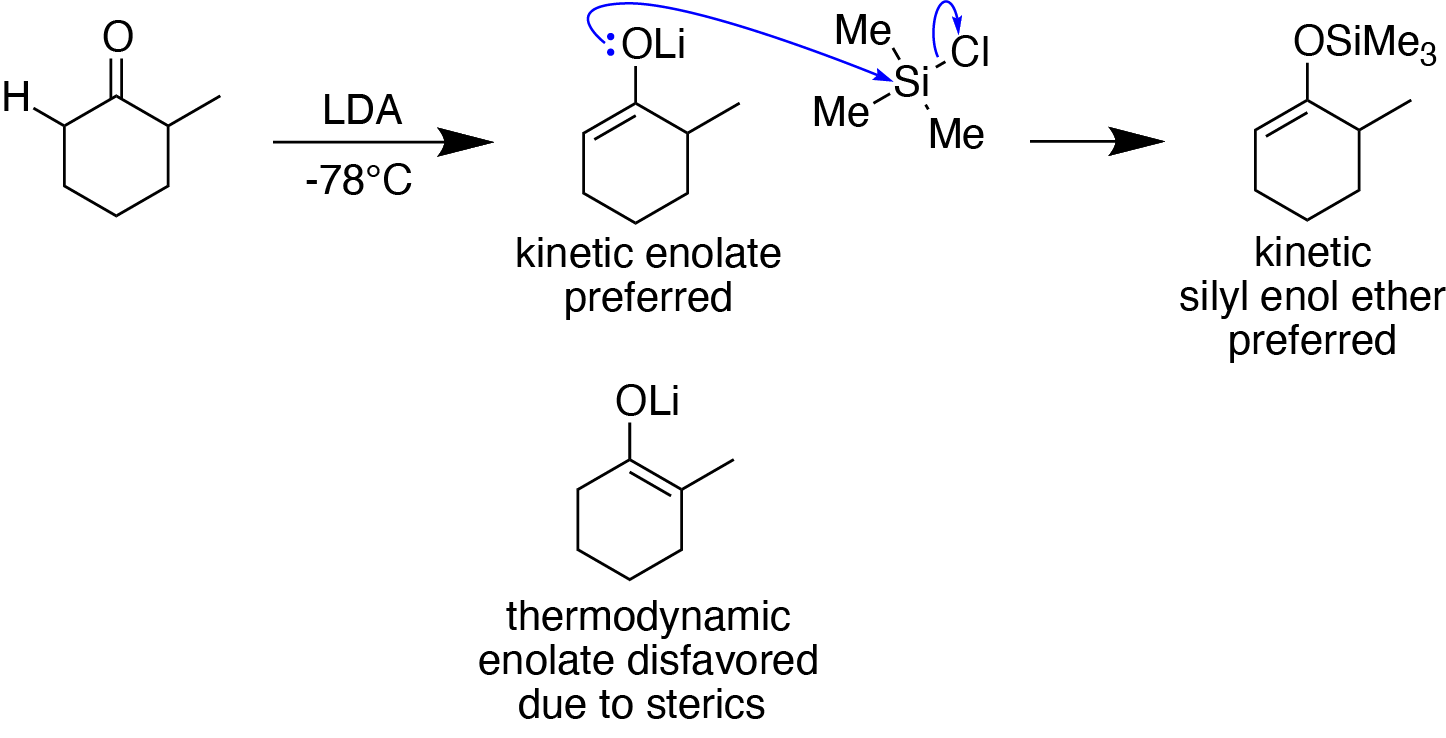
Enol Silyl Ethers
In organosilicon chemistry, silyl enol ethers are a class of organic compounds that share the common functional group , composed of an enolate () bonded to a silane () through its oxygen end and an ethene group () as its carbon end. They are important intermediates in organic synthesis. Synthesis Silyl enol ethers are generally prepared by reacting an enolizable carbonyl compound with a silyl electrophile and a base, or just reacting an enolate with a silyl electrophile.Clayden, J., Greeves, N., & Warren, S. (2012). Silyl enol ethers. In ''Organic chemistry'' (Second ed., pp. 466-467). Oxford University Press. Since silyl electrophiles are hard and silicon-oxygen bonds are very strong, the oxygen (of the carbonyl compound or enolate) acts as the nucleophile to form a Si-O single bond. The most commonly used silyl electrophile is trimethylsilyl chloride. To increase the rate of reaction, trimethylsilyl triflate may also be used in the place of trimethylsilyl chloride as a more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enolates
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds. Bonding and structure Enolate anions are electronically related to allyl anions. The anionic charge is delocalized over the oxygen and the two carbon sites. Thus they have the character of both an alkoxide and a carbanion. Although they are often drawn as being simple salts, in fact they adopt complicated structures often featuring aggregates. Preparation Deprotonation of enolizable ketones, aromatic alcohols, aldehydes, and esters gives enolates. With strong bases, the deprotonation is quantitative. Typically enolates are generated from using lithium diisopropylamide (LDA). Often, as in conventional Claisen condensations, Mannich reactions, and aldol condensations, enolates are generated in low concentrations with alkoxide bases. Under such conditions, they exist in low concen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |