Tosyl on:
[Wikipedia]
[Google]
[Amazon]

 In
In
 The tosyl group is also useful as a protecting group for
The tosyl group is also useful as a protecting group for
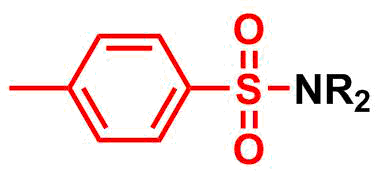 Tosyl (Ts) group is commonly used as a
Tosyl (Ts) group is commonly used as a
 * Refluxing with TMSCl,
* Refluxing with TMSCl,
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
with the chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
. It consists of a tolyl group, , joined to a sulfonyl group, , with the open valence on sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
. This group is usually derived from the compound tosyl chloride, (abbreviated TsCl), which forms ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s and amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s of toluenesulfonic acid, (abbreviated TsOH). The para orientation illustrated (''p''-toluenesulfonyl) is most common, and by convention ''tosyl'' without a prefix refers to the ''p''-toluenesulfonyl group.
The tosyl terminology was proposed by German chemists Kurt Hess and Robert Pfleger in 1933 on the pattern of trityl and adopted in English starting from 1934.
The toluenesulfonate (or tosylate) group refers to the (–OTs) group, with an additional oxygen attached to sulfur and open valence on an oxygen. In a chemical name, the term ''tosylate'' may either refer to the salts
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions (anions), which results in a compound with no net electric charge (electrically neutral). ...
containing the anion of ''p''-toluenesulfonic acid, (e.g., sodium p-toluenesulfonate), or it may refer to ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s of ''p''-toluenesulfonic acid, TsOR (R = organyl group
In organic and organometallic chemistry, an organyl group (commonly denoted by the letter " R") is an organic substituent with one (sometimes more) free valence electron(s) at a carbon atom.. The term is often used in chemical patent literatur ...
).
Applications
For S2 reactions, alkyl alcohols can also be converted to alkyl tosylates, often through addition of tosyl chloride. In this reaction, the lone pair of the alcohol oxygen attacks the sulfur of the tosyl chloride, displacing the chloride and forming the tosylate with retention of reactant stereochemistry. This is useful because alcohols are poor leaving groups in S2 reactions, in contrast to the tosylate group. It is the transformation of alkyl alcohols to alkyl tosylates that allows an S2 reaction to occur in the presence of a good nucleophile. A tosyl group can function as aprotecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In man ...
in organic synthesis. Alcohols can be converted to tosylate groups so that they do not react. The tosylate group may later be converted back into an alcohol. The use of these functional groups is exemplified in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
of the drug tolterodine, wherein one of the steps a phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
group is protected as its tosylate and the primary alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
as its nosylate. The latter is a leaving group for displacement by diisopropylamine:Reaction sequence: organic reduction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions car ...
of ''ethyl benzoylacetate'' by sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ...
to a diol, followed by Friedel-Crafts alkylation with p-cresol
''para''-Cresol, also 4-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of ''o''- ...
and iron(III) chloride to a phenol. The tosyl and nosyl groups are introduced as their respective chlorides with either sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
or triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
as a base. The next step is nucleophilic displacement of the nosyl group by diisopropylamine, the remaining tosyl group is removed by another round of NaOH. Not shown: optical resolution
Optical resolution describes the ability of an imaging system to resolve detail, in the object that is being imaged.
An imaging system may have many individual components, including one or more lenses, and/or recording and display components. E ...
by L-tartaric acid to optically pure (R)-isomer
: The tosyl group is also useful as a protecting group for
The tosyl group is also useful as a protecting group for amines
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
. The resulting sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this gro ...
structure is extremely stable. It can be deprotected to reveal the amine using reductive or strongly acidic conditions.
Amine protection – tosyl (Ts)
 Tosyl (Ts) group is commonly used as a
Tosyl (Ts) group is commonly used as a protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In man ...
for amines
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
.
Most common amine protection methods
* Tosyl chloride andpyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
in dichloromethane
Most common amine deprotection methods
* HBr andacetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
at 70 °C * Refluxing with TMSCl,
* Refluxing with TMSCl, sodium iodide
Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions ...
and acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
* Reduction with SmI2
* Reduction with Red-Al
Related compounds
Closely related to the tosylates are the nosylates and brosylates, which are the abbreviated names for ''o''- or ''p-''nitrobenzenesulfonates and ''p''-bromobenzenesulfonates, respectively.See also
* Tosylic acid * Sulfonyl groupNotes
References
{{Reflist Sulfonyl groups Leaving groups Sulfonate esters