|
Dihydroxylation
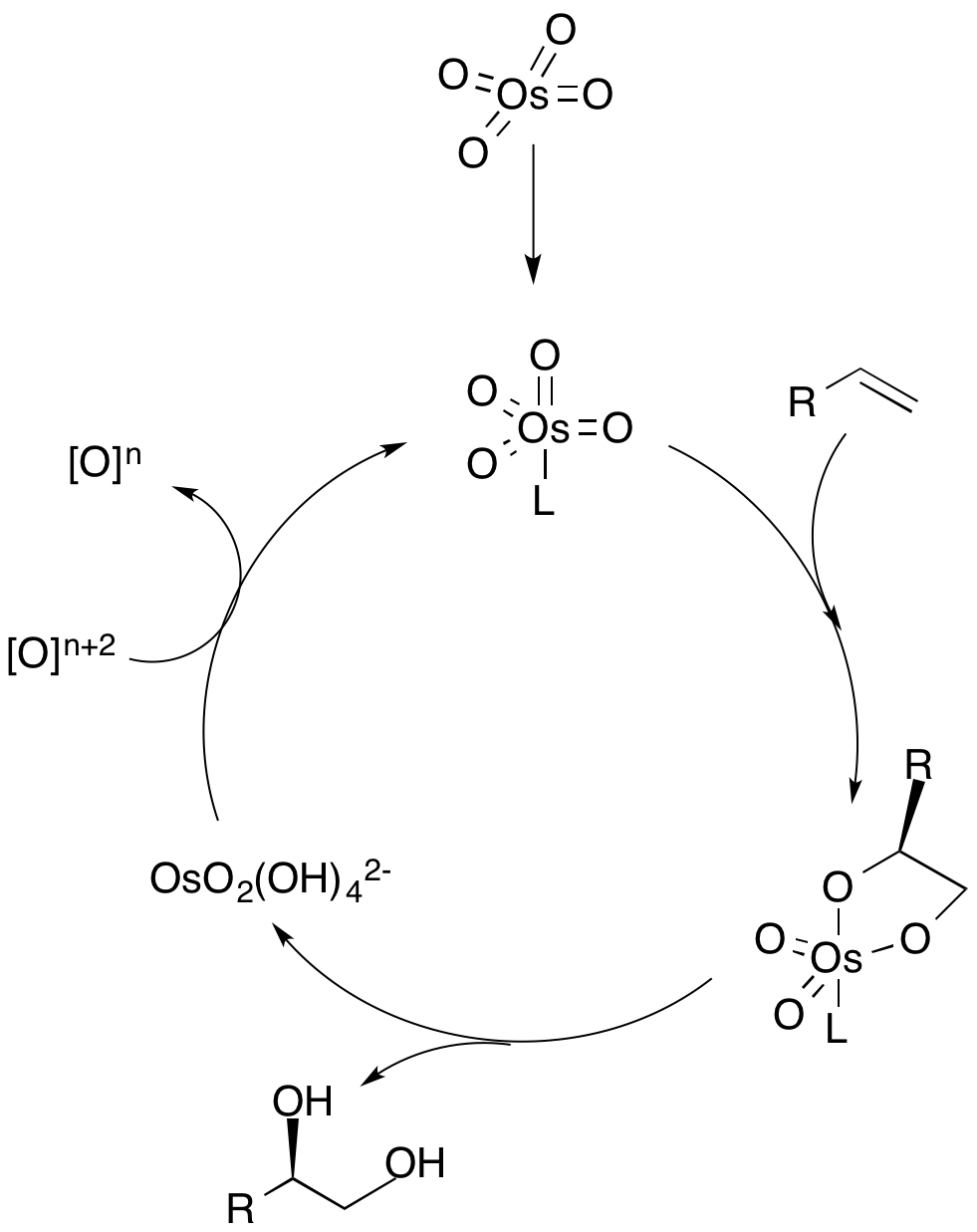
Dihydroxylation is the process by which an alkene is converted into a vicinal diol. Although there are many routes to accomplish this oxidation, the most common and direct processes use a high-oxidation-state transition metal (typically osmium or manganese). The metal is often used as a catalyst, with some other stoichiometric oxidant present. In addition, other transition metals and non-transition metal methods have been developed and used to catalyze the reaction. Mechanism In the dihydroxylation mechanism, a ligand first coordinates to the metal catalyst (depicted as osmium), which dictates the chiral selectivity of the olefin. The alkene then coordinates to the metal through a +2cycloaddition, and the ligand dissociates from the metal catalyst. Hydrolysis of the olefin then yields the vicinal diol, and oxidation of the catalyst by a stoichiometric oxidant regenerates the metal catalyst to repeat the cycle. The concentration of the olefin is crucial to the enantiomer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sharpless Asymmetric Dihydroxylation
Sharpless asymmetric dihydroxylation (also called the Sharpless bishydroxylation) is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol. The reaction has been applied to alkenes of virtually every substitution, often high enantioselectivities are realized, with the chiral outcome controlled by the choice of dihydroquinidine (DHQD) vs dihydroquinine (DHQ) as the ligand. Asymmetric dihydroxylation reactions are also highly site selective, providing products derived from reaction of the most electron-rich double bond in the substrate. It is common practice to perform this reaction using a catalytic amount of osmium tetroxide, which after reaction is regenerated with reoxidants such as potassium ferricyanide or ''N''-methylmorpholine ''N''-oxide. This dramatically reduces the amount of the highly toxic and very expensive osmium tetroxide needed. These four reagents are commercially available premixed (" AD ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydroxylation
Dihydroxylation is the process by which an alkene is converted into a vicinal diol. Although there are many routes to accomplish this oxidation, the most common and direct processes use a high-oxidation-state transition metal (typically osmium or manganese). The metal is often used as a catalyst, with some other stoichiometric oxidant present. In addition, other transition metals and non-transition metal methods have been developed and used to catalyze the reaction. Mechanism In the dihydroxylation mechanism, a ligand first coordinates to the metal catalyst (depicted as osmium), which dictates the chiral selectivity of the olefin. The alkene then coordinates to the metal through a +2cycloaddition, and the ligand dissociates from the metal catalyst. Hydrolysis of the olefin then yields the vicinal diol, and oxidation of the catalyst by a stoichiometric oxidant regenerates the metal catalyst to repeat the cycle. The concentration of the olefin is crucial to the enantiomer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmium Dihydroxylation Mechanism
Osmium (from Greek grc, ὀσμή, osme, smell, label=none) is a chemical element with the symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mostly in platinum ores. Osmium is the densest naturally occurring element. When experimentally measured using X-ray crystallography, it has a density of . Manufacturers use its alloys with platinum, iridium, and other platinum-group metals to make fountain pen nib tipping, electrical contacts, and in other applications that require extreme durability and hardness. Osmium is among the rarest elements in the Earth's crust, making up only 50 parts per trillion ( ppt). It is estimated to be about 0.6 parts per billion in the universe and is therefore the rarest precious metal. Characteristics Physical properties Osmium has a blue-gray tint and is the densest stable element; it is approximately twice as dense as lead and narrowly denser tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylation In The Synthesis Of Brassinosteroid
In chemistry, hydroxylation can refer to: *(i) most commonly, hydroxylation describes a chemical process that introduces a hydroxyl group () into an organic compound. *(ii) the ''degree of hydroxylation'' refers to the number of OH groups in a molecule. The ''pattern of hydroxylation'' refers to the location of hydroxy groups on a molecule or material. Hydroxylation reactions Synthetic hydroxylations Installing hydroxyl groups into organic compounds can be effected by various metal catalysts. Many such catalysts are biomimetic, i.e. they are inspired by or intended to mimic enzymes such as cytochrome P450. Whereas many hydroxylations insert O atoms into bonds, some reactions ''add'' OH groups to unsaturated substrates. The Sharpless dihydroxylation is such a reaction: it converts alkenes into diols. The hydroxy groups are provided by hydrogen peroxide, which adds across the double bond of alkenes. Biological hydroxylation In biochemistry, hydroxylation reactions are often f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Milas Hydroxylation
The Milas hydroxylation is an organic reaction converting an alkene to a vicinal diol, and was developed by Nicholas A. Milas in the 1930s. The cis-diol is formed by reaction of alkenes with hydrogen peroxide and either ultraviolet light or a catalytic osmium tetroxide, vanadium pentoxide, or chromium trioxide. The reaction has been superseded in synthetic chemistry by the Upjohn dihydroxylation and later by the Sharpless asymmetric dihydroxylation Sharpless asymmetric dihydroxylation (also called the Sharpless bishydroxylation) is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol. The reaction has been applied to alk .... Mechanism The proposed mechanism for the Milas hydroxylation involves the initial combination of hydrogen peroxide and the osmium tetroxide catalyst to form an intermediate, which then adds to the alkene, followed by a cleavage that forms the product and regenerates the OsO4. Li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Upjohn Dihydroxylation
The Upjohn dihydroxylation is an organic reaction which converts an alkene to a ''cis'' vicinal diol. It was developed by V. VanRheenen, R. C. Kelly and D. Y. Cha of the Upjohn Company in 1976. It is a catalytic system using ''N''-methylmorpholine ''N''-oxide (NMO) as stoichiometric re-oxidant for the osmium tetroxide. It is superior to previous catalytic methods. Prior to this method, use of stoichiometric amounts of the toxic and expensive reagent osmium tetroxide was often necessary. The Upjohn dihydroxylation is still often used for the formation of ''cis''-vicinal diols; however, it can be slow and is prone to ketone byproduct formation. One of the peculiarities of the dihydroxylation of olefins is that the standard "racemic" method (the Upjohn dihydroxylation) is slower and often lower yielding than the asymmetric method (the Sharpless asymmetric dihydroxylation). Improvements to Upjohn dihydroxylation In response to these problems, Stuart Warren and co-workers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prevost And Woodward First Steps
Prevost, Prévost or Prévôt may refer to: Places * Prévost (electoral district), Quebec, a provincial electoral district * Prévost, Quebec, a community in the Laurentians region of Quebec, Canada ** Prévost station * Prevost, a community on Stuart Island, San Juan County, Washington, USA Ships *HMCS Prevost, a Canadian naval reserve unit in London, Ontario *, a 12-gun schooner that the Royal Navy purchased in 1803 and that the French privateer ''Austerlitz'' captured in 1807 * HMS ''Sir George Prevost'', a British naval warship * USS ''Lady Prevost'' (1812), a United States warship Other uses *Prevost Car, a bus manufacturer and division of Volvo Buses *Prévost reaction, a chemical reaction *Prevost's ground sparrow, a sparrow *Prevost's squirrel, a rodent People with the surname *Abbé Prévost (1697–1763), French novelist *André Prévost (composer) (1934–2001), Canadian composer *Augustine Prévost (1723–1786), British general *Charles Prévost (1899–1983), Fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Woodward Reaction
A woodward is a warden of a wood. Woodward may also refer to: Places ;United States * Woodward, Iowa * Woodward, Oklahoma * Woodward, Pennsylvania, a census-designated place * Woodward Avenue, a street in Tallahassee, Florida, which bisects the campus of Florida State University * Woodward Avenue, a Michigan state highway * Woodward Corridor, a neighborhood in Detroit, Michigan * Woodward County, Oklahoma * Woodward Park (other), multiple places * Woodward Pond, a man-made pond in Bowie, Maryland * Woodward Township, Pennsylvania (other), multiple places People * Woodward (surname) * Frank Lee Woodward (1871–1952), English educationist, Pali scholar, author and theosophist Businesses * Woodward, Inc., American maker of energy devices * Woodward & Lothrop, American department store chain * Woodward Iron Company, in Birmingham (Woodward) Alabama * Woodward's, Canadian department store chain ** The Woodward's building in Vancouver, British Columbia Education * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydropyran
In organic chemistry, dihydropyran refers to two heterocyclic compounds with the formula C5H8O: * 3,4-Dihydro-2''H''-pyran *3,6-dihydro-2''H''-pyran Nomenclature In IUPAC names, "dihydro" refers to the two added hydrogen atoms needed to remove one double bond from the parent compound pyran. The numbers in front of the prefix indicate the position of the added hydrogen atoms (and not the position of the double bonds). The italicized capital ''H'' denotes the "indicated hydrogen", which is a second hydrogen atom present on the location where no double bond is present.A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993)''R-1.3 Indicated Hydrogen''/ref> See also *Pyran *Tetrahydropyran Tetrahydropyran (THP) is the organic compound consisting of a saturated six-membered ring containing five carbon atoms and one oxygen atom. It is named by reference to pyran, which contains two double bonds, and may be produced from it by addi ... References {{DEFAULTS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
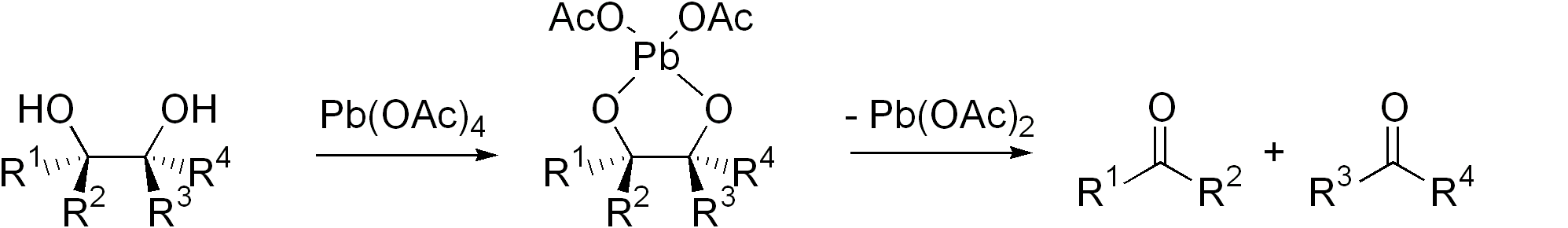
Glycol Cleavage
Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a vicinal diol (glycol) is cleaved and instead the two oxygen atoms become double-bonded to their respective carbon atoms. Depending on the substitution pattern in the diol, these carbonyls can be either ketones or aldehydes. Glycol cleavage is an important reaction in the laboratory because it is useful for determining the structures of sugars. After cleavage takes place the ketone and aldehyde fragments can be inspected and the location of the former hydroxyl groups ascertained. Reagents Periodic acid (HIO4), (diacetoxyiodo)benzene (PhI(OAc)2) and lead tetraacetate (Pb(OAc)4) are the most common reagents used for glycol cleavage, processes called the Malaprade reaction and Criegee oxidation, respectively. These reactions are most efficient when a cyclic intermediate can form, with the iodine or lead atom linking both oxygen atoms. The ring then fragments, with breakage of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |