Glycol Cleavage on:
[Wikipedia]
[Google]
[Amazon]
Glycol cleavage is a specific type of organic chemistry
 Warm concentrated
Warm concentrated
www.cem.msu.edu
{{Alcohols Organic redox reactions
oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
. The carbon–carbon bond in a vicinal diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting gro ...
(glycol) is cleaved and instead the two oxygen atoms become double-bonded to their respective carbon atoms. Depending on the substitution pattern in the diol, these carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
s will be ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s and/or aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s.
Glycol cleavage is an important for determining the structures of sugars. After cleavage of the glycol, the ketone and aldehyde fragments can be inspected and the location of the former hydroxyl groups ascertained.
Reagents
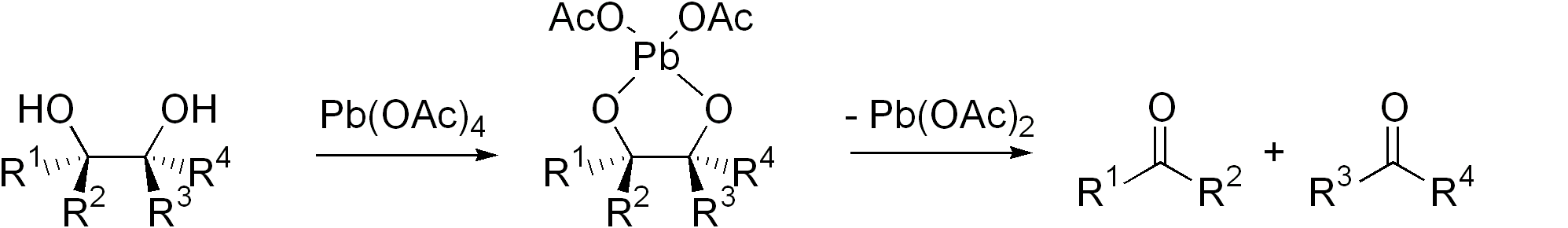
Iodine-based reagents such asperiodic acid
Periodic acid ( ) is an oxoacid of iodine. It can exist in two forms: orthoperiodic acid, with the chemical formula , and metaperiodic acid, which has the formula . Periodic acids are colourless crystals. Periodic acid features iodine in the hig ...
(HIO4) and (diacetoxyiodo)benzene (PhI(OAc)2) are commonly used. Another reagent is lead tetraacetate (Pb(OAc)4). These I- and Pb-based methods are called the Malaprade reaction and Criegee oxidation, respectively. The former is favored for aqueous solutions, the latter for nonaqueous solutions.
Cyclic intermediate are invariably invoked. The ring then fragments, with cleavage of the carbon–carbon bond and formation of carbonyl groups.
: Warm concentrated
Warm concentrated potassium permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, which dissolves in water as K+ and ions to give an intensely pink to purple solution.
Potassium permanganate is widely us ...
(KMnO4) will react with an alkene to form a glycol. Following this dihydroxylation
Dihydroxylation is the process by which an alkene is converted into a vicinal (chemistry), vicinal diol. Although there are many routes to accomplish this oxidation, the most common and direct processes use a high-oxidation-state transition metal ...
, the KMnO4 can then cleave the glycol to give aldehydes or ketones. The aldehydes will react further with (KMnO4), being oxidized to become carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s. Controlling the temperature, concentration of the reagent and the pH of the solution can keep the reaction from continuing past the formation of the glycol.
References
External links
www.cem.msu.edu
{{Alcohols Organic redox reactions