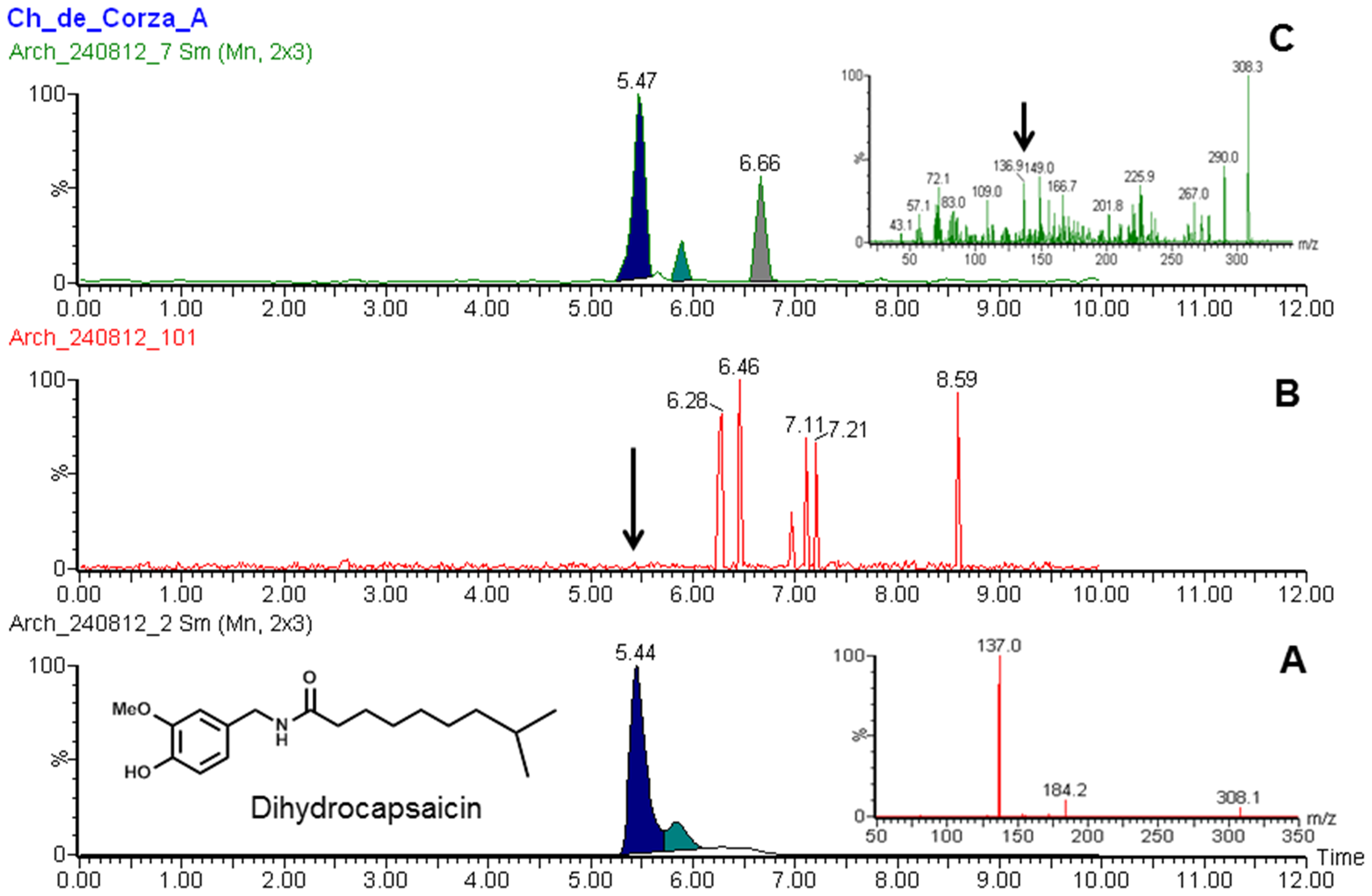
|
Dihydrocapsaicin
Dihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (''Capsicum''). Like capsaicin, it is an irritant. It accounts for about 22% of the total capsaicinoid mixture and has the same pungency as capsaicin. Pure dihydrocapsaicin is a lipophilic colorless odorless crystalline to waxy compound. It is soluble in dimethyl sulfoxide and 100% ethanol. See also * Capsaicin * Nordihydrocapsaicin * Homocapsaicin * Homodihydrocapsaicin * Nonivamide * Scoville scale * Pepper spray * Hot sauce Hot sauce is a type of condiment, seasoning, or salsa (sauce), salsa made from chili peppers and other ingredients. Many commercial varieties of Mass production, mass-produced hot sauce exist. History Humans have used chili peppers and other ho ... References External links Molecule of the MonthSafety MSDS data {{Transient receptor potential channel modulators Capsaicinoids Acetamides Methoxy compounds Transient receptor potential channel modu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capsaicin
Capsaicin (8-methyl-''N''-vanillyl-6-nonenamide) (, rarely ) is an active component of chili peppers, which are plants belonging to the genus ''Capsicum''. It is a potent Irritation, irritant for Mammal, mammals, including humans, and produces a sensation of burning in any Tissue (biology), tissue with which it comes into contact. Capsaicin and several related amides (capsaicinoids) are produced as secondary metabolites by chili peppers, likely as deterrents against certain mammals and fungi. Pure capsaicin is a hydrophobic, colorless, highly pungent (i.e., spicy) crystalline solid. Natural function Capsaicin is present in large quantities in the Placentation#In plants, placental tissue (which holds the seeds), the internal membranes and, to a lesser extent, the other fleshy parts of the fruits of plants in the genus ''Capsicum''. The seeds themselves do not produce any capsaicin, although the highest concentration of capsaicin can be found in the white Fruit anatomy#Mesocar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capsaicinoids
Capsaicin (8-methyl-''N''-vanillyl-6-nonenamide) (, rarely ) is an active component of chili peppers, which are plants belonging to the genus ''Capsicum''. It is a potent irritant for mammals, including humans, and produces a sensation of burning in any tissue with which it comes into contact. Capsaicin and several related amides (capsaicinoids) are produced as secondary metabolites by chili peppers, likely as deterrents against certain mammals and fungi. Pure capsaicin is a hydrophobic, colorless, highly pungent (i.e., spicy) crystalline solid. Natural function Capsaicin is present in large quantities in the placental tissue (which holds the seeds), the internal membranes and, to a lesser extent, the other fleshy parts of the fruits of plants in the genus ''Capsicum''. The seeds themselves do not produce any capsaicin, although the highest concentration of capsaicin can be found in the white pith of the inner wall, where the seeds are attached. The seeds of ''Capsicum'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scoville Scale
The Scoville scale is a measurement of spiciness of chili peppers and other substances, recorded in Scoville heat units (SHU). It is based on the concentration of capsaicinoids, among which capsaicin is the predominant component. The scale is named after its creator, American pharmacist Wilbur Scoville, whose 1912 method is known as the Scoville organoleptic test. The Scoville organoleptic test is a subjective assessment derived from the capsaicinoid sensitivity by people experienced with eating hot chilis. An alternative method, high-performance liquid chromatography (HPLC), can be used to analytically quantify the capsaicinoid content as an indicator of pungency. Scoville organoleptic test In the Scoville organoleptic test, an exact weight of dried pepper is dissolved in alcohol to extract the heat components (capsaicinoids), then diluted in a solution of sugar water. Decreasing concentrations of the extracted capsaicinoids are given to a panel of five trained tasters, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homodihydrocapsaicin
Homodihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (''Capsicum''). Like capsaicin it is an irritant. Homodihydrocapsaicin accounts for about 1% of the total capsaicinoids mixture and has about half the pungency of capsaicin. Pure homodihydrocapsaicin is a lipophilic colorless odorless crystalline to waxy compound. It produces "numbing burn" in the throat and is one of the most prolonged and difficult to rinse out. On the Scoville scale it has 8,600,000 SHU (Scoville heat units). See also * Capsaicin * Dihydrocapsaicin * Nordihydrocapsaicin * Homocapsaicin * Nonivamide * Scoville scale * Pepper spray * Spice In the culinary arts, a spice is any seed, fruit, root, Bark (botany), bark, or other plant substance in a form primarily used for flavoring or coloring food. Spices are distinguished from herbs, which are the leaves, flowers, or stems of pl ... References External links Molecule of the Month {{Transient recepto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homocapsaicin
Homocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (''Capsicum''). Like capsaicin it is an irritant. Homocapsaicin accounts for about 1% of the total capsaicinoids mixture and has about half the pungency of capsaicin. Pure homocapsaicin is a lipophilic colorless odorless crystalline to waxy compound. On the Scoville scale it has 8,600,000 SHU (''Scoville heat units''). Homocapsaicin isolated from chili pepper has been found in two isomeric forms, both with a carbon-carbon double bond at the 6 position (numbered from the amide carbon) on the 10-carbon acyl chain. One isomer has an additional carbon, a methyl group, at the 8 position and the other has a methyl group at the 9 position. Homocapsaicin (6-ene-8-methyl) is the more abundant isomer. Homocapsaicin with the double bond at the 7 position has never been found in nature, though its structure is widely reported on the Internet and in the scientific literature. Details of this misidentificatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nordihydrocapsaicin
Nordihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (''Capsicum''). Properties Like capsaicin, it is an irritant. Nordihydrocapsaicin accounts for about 7% of the total capsaicinoids mixture and has about half the pungency of capsaicin. Pure nordihydrocapsaicin is a lipophilic colorless odorless crystalline to waxy solid. On the Scoville scale it has 9,100,000 SHU (''Scoville heat units''), significantly higher than pepper spray. See also * Capsaicin * Dihydrocapsaicin * Homocapsaicin * Homodihydrocapsaicin * Nonivamide * Scoville scale * Pepper spray * Spice In the culinary arts, a spice is any seed, fruit, root, Bark (botany), bark, or other plant substance in a form primarily used for flavoring or coloring food. Spices are distinguished from herbs, which are the leaves, flowers, or stems of pl ... References Capsaicinoids Acetamides {{Ether-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula . This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO is metabolised to compounds that leave a garlic-like taste in the mouth after DMSO is absorbed by skin. In terms of chemical structure, the molecule has idealized Cs symmetry. It has a trigonal pyramidal molecular geometry consistent with other three-coordinate S(IV) compounds, with a nonbonded electron pair on the approximately tetrahedral sulfur atom. Synthesis and production Dimethyl sulfoxide was first synthesized in 1866 by the Russian scientist Alexander Zaytsev, who reported his findings in 1867. Its modern use as an industrial solvent began through popularization by Thor Smedslund at the Stepan Chemical Company. Dimeth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Congener (chemistry)
In chemistry, congeners are chemical substances "related to each other by origin, structure, or function". Common origin and structure Any significant quantity of a polyhalogenated compound is by default a blend of multiple molecule types because each molecule forms independently, and chlorine and bromine do not strongly select which site(s) they bond to. *Polychlorinated biphenyls (PCBs) are a family of 209 congeners. * Polybrominated biphenyls and polychlorinated diphenyl ethers are also families of 209 congeners. Similarly polychlorinated dibenzodioxins, polychlorinated dibenzofurans, polychlorinated terphenyls, polychlorinated naphthalene, polychloro phenoxy phenol, and polybrominated diphenyl ethers (PBDEs) ( pentabromodiphenyl ether, octabromodiphenyl ether, decabromodiphenyl ether), etc. are also groups of congeners. Common origin * Congener (alcohol), substances other than alcohol (desirable or undesirable) also produced during fermentation. *Congeners of olei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipophilic
Lipophilicity (from Greek language, Greek λίπος "fat" and :wikt:φίλος, φίλος "friendly") is the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such compounds are called lipophilic (translated as "fat-loving" or "fat-liking"). Such non-polar solvents are themselves lipophilic, and the adage "like dissolves like" generally holds true. Thus lipophilic substances tend to dissolve in other lipophilic substances, whereas hydrophilic ("water-loving") substances tend to dissolve in water and other hydrophilic substances. Lipophilicity, hydrophobicity, and non-polarity may describe the same tendency towards participation in the London dispersion force, as the terms are often used interchangeably. However, the terms "lipophilic" and "hydrophobicity, hydrophobic" are not synonymous, as can be seen with silicones and fluorocarbons, which are hydrophobic but not lipophilic. __TOC__ Surfactants Hydrocarbon-based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |