|
Diazonaphthoquinone
Diazonaphthoquinone (DNQ) is a diazo derivative of naphthoquinone. Upon exposure to light, DNQ converts to a derivative that is susceptible to etching. In this way, DNQ has become an important reagent in photoresist technology in the semiconductor industry. Diazonaphthoquinone sulfonic acid esters are components of common photoresist materials. Such photoresists are used in the manufacture of semiconductors. In this application DNQs are mixed with Novolac resin, a type of phenolic polymer. The DNQ functions as a dissolution inhibitor. During the masking/patterning process, portions of the photoresist film are exposed to light while others remain unexposed. In the unexposed regions of the resist film, the DNQ acts as a dissolution inhibitor and the resist remains insoluble in the aqueous base developer. In the exposed regions, the DNQ forms a ketene In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), mon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronics industry. The process begins by coating a substrate with a light-sensitive organic material. A patterned mask is then applied to the surface to block light, so that only unmasked regions of the material will be exposed to light. A solvent, called a developer, is then applied to the surface. In the case of a positive photoresist, the photo-sensitive material is degraded by light and the developer will dissolve away the regions that were exposed to light, leaving behind a coating where the mask was placed. In the case of a negative photoresist, the photosensitive material is strengthened (either polymerized or cross-linked) by light, and the developer will dissolve away only the regions that were not exposed to light, leaving behind a coating in areas w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazo
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes and are described by the general structural formula . The simplest example of a diazo compound is diazomethane, . Diazo compounds () should not be confused with azo compounds () or with diazonium compounds (). Structure The electronic structure of diazo compounds is characterized by π electron density delocalized over the α-carbon and two nitrogen atoms, along with an orthogonal π system with electron density delocalized over only the terminal nitrogen atoms. Because all octet rule-satisfying resonance forms of diazo compounds have formal charges, they are members of a class of compounds known as 1,3-dipoles. Some of the most stable diazo compounds are α-diazo-β-diketones and α-diazo-β-diesters, in which the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthoquinone
Naphthoquinones constitute a class of organic compounds structurally related to naphthalene. Two isomers are common for the parent naphthoquinones: * 1,2-Naphthoquinone * 1,4-Naphthoquinone Natural products * Alkannin * Hexahydroxy-1,4-naphthalenedione * Juglone * Lapachol * Lawsone * Menatetrenone * 2-Methoxy-1,4-naphthoquinone, a compound found in '' Impatiens'' species * Nigrosporin B * Phylloquinone * Plumbagin * Spinochrome B * Spinochrome D * Vitamin K and related compounds ** Phylloquinone ** Vitamin K2 ** Menadione Menadione is a natural organic compound with the formula C6H4(CO)2C2H(CH3). It is an analog of 1,4-naphthoquinone with a methyl group in the 2-position. It is sometimes called vitamin K3. Use is allowed as a nutritional supplement in animal ... (2-Methyl-1,4-naphthoquinone) Synthetic naphthoquinones * 5,8-Dihydroxy-1,4-naphthoquinone and dihydroxynaphthoquinones * Atovaquone * Buparvaquone, an antiprotozoal drug used in veterinary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Novolac
Novolaks (sometimes: novolacs) are low molecular weight polymers derived from phenols and formaldehyde. They are related to Bakelite, which is more highly crosslinked. The term comes from Swedish "lack" for lacquer and Latin "novo" for new, since these materials were envisioned to replace natural lacquers such as copal resin. Typically novolaks are prepared by the condensation of phenol or a mixture of p- and m-cresol with formaldehyde (as formalin). The reaction is acid catalyzed. Oxalic acid is often used because it can be subsequently removed by thermal decomposition. Novolaks have a degree of polymerization of approximately 20-40. The branching density, determined by the processing conditions, m- vs p-cresol ratio, as well as CH2O/cresol ratio is typically around 15%. Novolaks are especially important in microelectronics where they are used as photoresist materials. They are also used as tackifiers in rubber. See also * Phenol formaldehyde resin * Epoxy Epoxy is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. It is acutely toxic and is considered a health hazard. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 million tonnes a year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds, and is a liquid when manufactured. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, explosives such as picric acid, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceuti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compound (chemistry), compounds, produces unique physical property, physical properties including toughness, high rubber elasticity, elasticity, viscoelasticity, and a tendency to form Amorphous solid, amorphous and crystallization of polymers, semicrystalline structures rath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
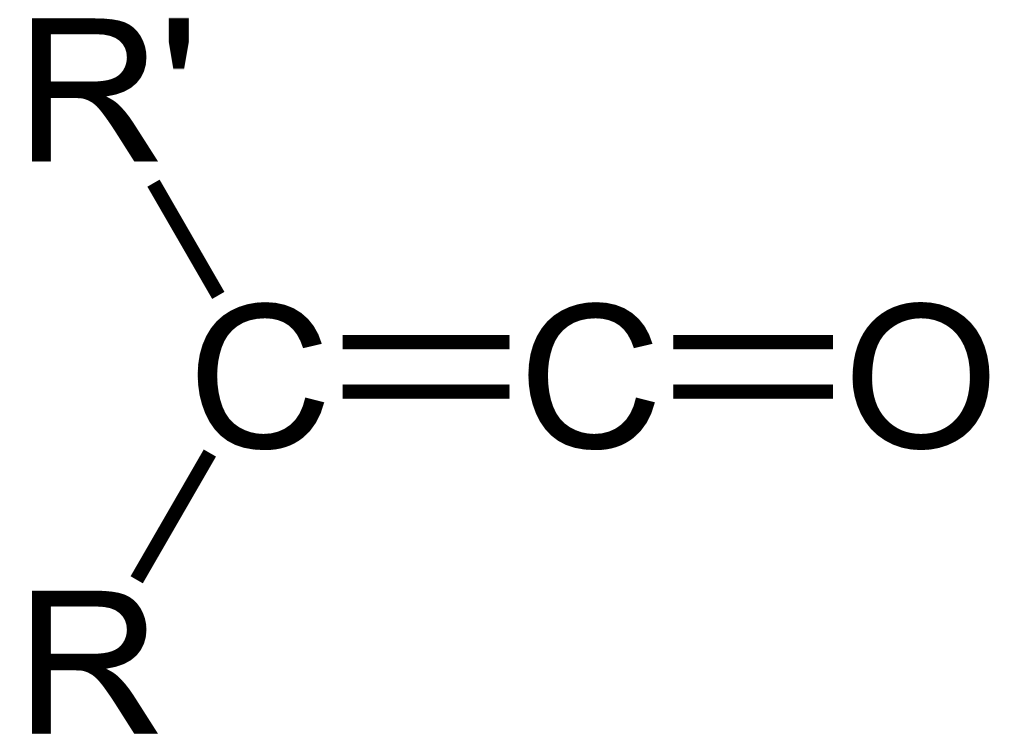
Ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are chemical stability, unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
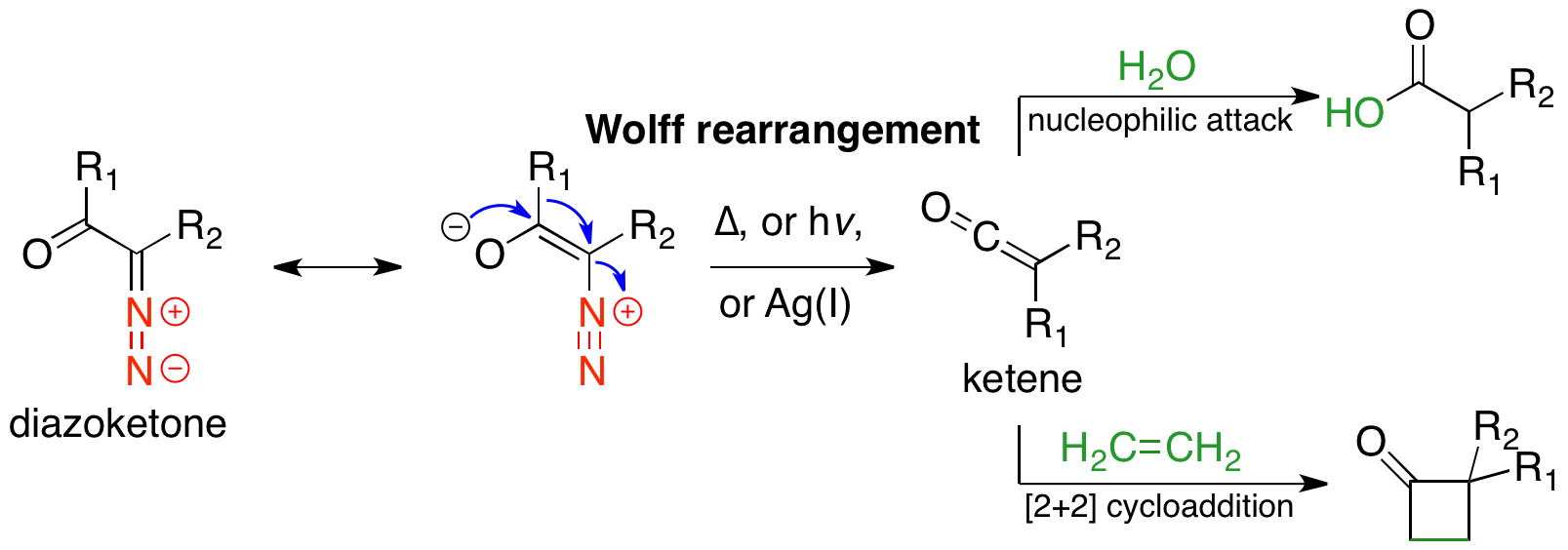
Wolff Rearrangement
The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weak acids, weakly acidic nucleophiles such as water, alcohols, and amines, to generate carbonyl#carboxylic acid derivatives, carboxylic acid derivatives or undergo 2+2 Photocycloaddition, [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indene
Indene is an aromatic, polycyclic hydrocarbon with chemical formula . It is composed of a benzene ring fused with a cyclopentene ring. This flammable liquid is colorless although samples often are pale yellow. The principal industrial use of indene is in the production of indene/ coumarone thermoplastic resins. Substituted indenes and their closely related indane derivatives are important structural motifs found in many natural products and biologically active molecules, such as sulindac. Isolation Indene occurs naturally in coal-tar fractions boiling around 175–185 °C. It can be obtained by heating this fraction with sodium to precipitate solid "sodio-indene". This step exploits indene's weak acidity evidenced by its deprotonation by sodium to give the indenyl derivative. The sodio-indene is converted back to indene by steam distillation. Reactivity Indene readily polymerises. Oxidation of indene with acid dichromate yields homophthalic acid (''o''-carboxylpheny ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |