|
Diazomethane Preparation - Macro Diazald Kit
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane. Use For safety and convenience diazomethane is always prepared as needed as a solution in diethyl ether, ether and used as such. It converts carboxylic acids to methyl esters and phenols into their methyl ethers. The reaction is thought to proceed via proton transfer from carboxylic acid to diazomethane to give a methyldiazonium cation, which reacts with the carboxylate ion to give th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyldiazonium
Methyldiazonium is an organic compound consisting of a methyl group attached to a diazo group. This cation is the conjugate acid of diazomethane, with an estimated p''K''a<10. : 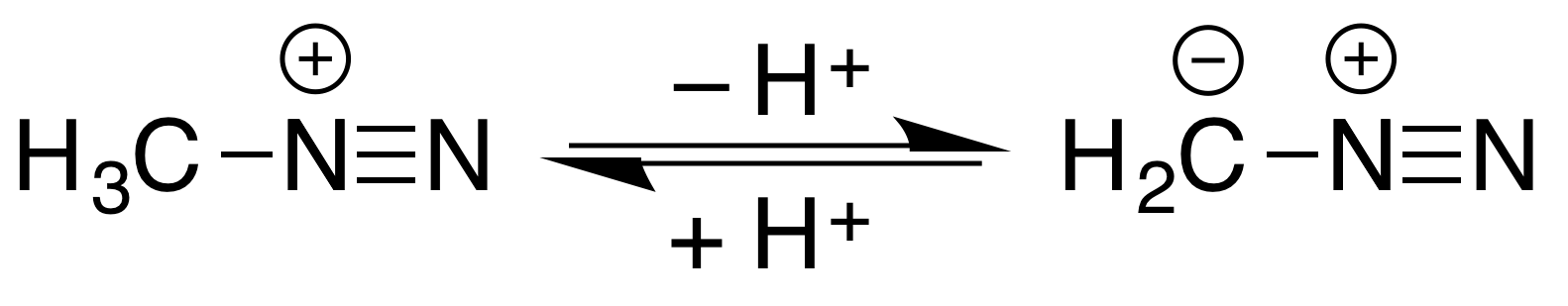 It is an intermediate in methylation reactions of diazomethane with acidic hydroxyl compounds, such as conversion of s to methyl esters and
It is an intermediate in methylation reactions of diazomethane with acidic hydroxyl compounds, such as conversion of s to methyl esters and
|
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazomethane Synthesis V
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane. Use For safety and convenience diazomethane is always prepared as needed as a solution in ether and used as such. It converts carboxylic acids to methyl esters and phenols into their methyl ethers. The reaction is thought to proceed via proton transfer from carboxylic acid to diazomethane to give a methyldiazonium cation, which reacts with the carboxylate ion to give the methyl es ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazald
Diazald (''N''-methyl-''N''-nitroso-''p''-toluenesulfonamide) is used as a relatively safe and easily handled precursor to diazomethane, which is toxic and unstable. Diazald has become the favored commercially available precursor for the synthesis of diazomethane, compared to reagents like N-Nitroso-N-methylurea, ''N''-methyl''-N''-nitrosourea and Methylnitronitrosoguanidine, ''N''-methyl-''N'''-nitro-''N''-nitrosoguanidine, which are less thermally stable and more toxic and mutagenic, respectively. Upon the addition of a Base (chemistry), base such as sodium hydroxide or potassium hydroxide and mild heating (65–70 °C) in a mixture of water, diethyl ether, and a high boiling polar cosolvent (e.g., 2-(2-Methoxyethoxy)ethanol, diethylene glycol monomethyl ether), the ''N''-nitrososulfonamide undergoes successive elimination reactions to produce diazomethane (which is codistilled as an ethereal solution) as well as a P-Toluenesulfonic acid, ''p''-toluenesulfonate salt as a byp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


