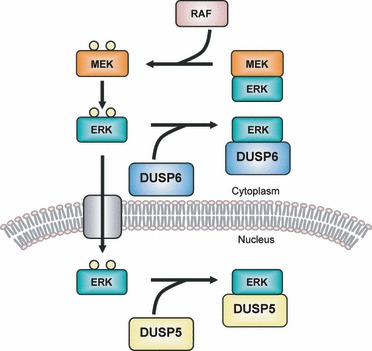
|
DUSP
Dual-specificity phosphatase (DUSP; DSP) is a form of phosphatase that can act upon tyrosine or serine/threonine residues. There are several families of dual-specificity phosphatase enzymes in mammals. All share a similar catalytic mechanism, by which a conserved cysteine residue forms a covalent intermediate with the phosphate group to be eliminated. The residues surrounding their catalytic core obey a rather strict consensus: His-Cys-x-x-x-x-x-Arg-Ser. The serine side chain and an additional conserved aspartate play a central role in the elimination of the Cys-linked intermediate, thus completing their enzymatic cycle. The main difference between tyrosine-specific phosphatases and dual-specificity phosphatases lies in the width of the latter enzymes' catalytic pocket: thus they can accommodate phosphorylated serine or threonine side chains as well as phosphorylated tyrosines. Classification The human genome encodes at least 61 different DUSP proteins. The following major group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MAPK Phosphatase
MAPK phosphatases (MKPs) are the largest class of phosphatases involved in down-regulating Mitogen-activated protein kinases (MAPK) signaling. MAPK Cell signaling, signalling pathways regulate multiple features of Developmental biology, development and homeostasis. This can involve gene regulation, cell proliferation, programmed cell death and stress responses. MAPK phosphatases are therefore important regulator components of these pathways. Function MAPK phosphatases are only found in eukaryotes and negatively regulate MAP kinases to act as negative feedback. MKPs are also known as dual-specificity phosphatases (DUSPs) because they deactivate MAPK by Dephosphorylation, dephosphorylating the Threonine and the Tyrosine residues residing in MAPKs activation site. MKPs have a Active site, catalytic region at their C-terminus and a Regulatory site, regulatory region at their N-terminus. The position where the MAPK binds to MKP is found near the N-terminus of MKP. The binding is due to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatase
In biochemistry, a phosphatase is an enzyme that uses water to cleave a phosphoric acid Ester, monoester into a phosphate ion and an Alcohol (chemistry), alcohol. Because a phosphatase enzyme catalysis, catalyzes the hydrolysis of its Substrate (chemistry), substrate, it is a subcategory of hydrolases. Phosphatase enzymes are essential to many biological functions, because phosphorylation (e.g. by protein kinases) and dephosphorylation (by phosphatases) serve diverse roles in cell growth, cellular regulation and cell signaling, signaling. Whereas phosphatases remove phosphate groups from molecules, kinases catalyze the transfer of phosphate groups to molecules from Adenosine triphosphate, ATP. Together, kinases and phosphatases direct a form of post-translational modification that is essential to the cell's regulatory network. Phosphatase enzymes are not to be confused with phosphorylase enzymes, which catalyze the transfer of a phosphate group from hydrogen phosphate to an acce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA. The one-letter symbol Y was assigned to tyrosine for being alphabetically nearest of those letters available. Note that T was assigned to the structurally simpler threonine, U was avoided for its similarity with V for valine, W was assigned to tryptophan, while X was reserved for undetermined or atypical amino acids. The mnemonic t''Y''rosine was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the proteinogenic amino acids. Only the L- stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, '' sericum''. Serine's structure was established in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− form when dissolved in water), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can und ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, but both D and L-cysteine are found in nature. LCysteine is a protein monomer in all biota, and D-cysteine acts as a signaling molecule in mammalian nervous systems. Cysteine is named after its discovery in urine, which comes from the urinary bladder or cyst, from Ancient Greek, Greek κύστις ''kýstis'', "bladder". The thiol is susceptible to oxidation to give the disulfide bond, disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. The deprotonated form can generally be described by the symbol Cym as well. When used as a food additive, cysteine has the E number E920. Cysteine is Genetic code, encoded by the codo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of proteins. D-aspartic acid is one of two D-amino acids commonly found in mammals. Apart from a few rare exceptions, D-aspartic acid is not used for protein synthesis but is incorporated into some peptides and plays a role as a neurotransmitter/ neuromodulator. Like all other amino acids, aspartic acid contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine-specific Phosphatase
Protein tyrosine phosphatases (EC 3.1.3.48, systematic name protein-tyrosine-phosphate phosphohydrolase) are a group of enzymes that remove phosphate groups from phosphorylated tyrosine residues on proteins: : proteintyrosine phosphate + H2O = proteintyrosine + phosphate Protein tyrosine (pTyr) phosphorylation is a common post-translational modification that can create novel recognition motifs for protein interactions and cellular localization, affect protein stability, and regulate enzyme activity. As a consequence, maintaining an appropriate level of protein tyrosine phosphorylation is essential for many cellular functions. Tyrosine-specific protein phosphatases (PTPase; ) catalyse the removal of a phosphate group attached to a tyrosine residue, using a cysteinyl-phosphate enzyme intermediate. These enzymes are key regulatory components in signal transduction pathways (such as the MAP kinase pathway) and cell cycle control, and are important in the control of cell growth, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylated Serine
Phosphoserine (abbreviated as SEP or J) is an ester of serine and phosphoric acid. Phosphoserine is a component of many proteins as the result of posttranslational modifications. The phosphorylation of the alcohol functional group in serine to produce phosphoserine is catalyzed by various types of kinases. Through the use of technologies that utilize an expanded genetic code, phosphoserine can also be incorporated into proteins during translation. It is a normal metabolite In biochemistry, a metabolite is an intermediate or end product of metabolism. The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ... found in human biofluids. Phosphoserine has three potential coordination sites (carboxyl, amine and phosphate group) Determination of the mode of coordination between phosphorylated ligands and metal ions occurring in an organism is a first step to explain the fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SSH1
''For the SSH-1 protocol, see Secure Shell#Version 1'' Protein phosphatase Slingshot homolog 1 is an enzyme that in humans is encoded by the ''SSH1'' gene In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei .... The ADF (actin-depolymerizing factor)/cofilin family (see MIM 601442) is composed of stimulus-responsive mediators of actin dynamics. ADF/cofilin proteins are inactivated by kinases such as LIM domain kinase-1 (LIMK1; MIM 601329). The SSH family appears to play a role in actin dynamics by reactivating ADF/cofilin proteins in vivo (Niwa et al., 2002). upplied by OMIMref name="entrez"/> References Further reading * * * * * * * * * * * * * * * * * {{gene-12-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SSH2
Protein phosphatase Slingshot homolog 2 is an enzyme that in humans is encoded by the ''SSH2'' gene In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei .... The ADF (actin-depolymerizing factor)/cofilin family (see MIM 601442) is composed of stimulus-responsive mediators of actin dynamics. ADF/cofilin proteins are inactivated by kinases such as LIM domain kinase-1 (LIMK1; MIM 601329). The SSH family appears to play a role in actin dynamics by reactivating ADF/cofilin proteins in vivo (Niwa et al., 2002). upplied by OMIMref name="entrez"> References Further reading * * * * * * * * * * External links * {{gene-17-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SSH3
Protein phosphatase Slingshot homolog 3 is an enzyme that in humans is encoded by the ''SSH3'' gene. Function The ADF (actin-depolymerizing factor)/cofilin family (see MIM 601442) is composed of stimulus-responsive mediators of actin dynamics. ADF/cofilin proteins are inactivated by kinases such as LIM domain kinase-1 (LIMK1 LIM domain kinase 1 is an enzyme that in humans is encoded by the ''LIMK1'' gene. Function There are approximately 40 known eukaryotic LIM proteins, so named for the LIM domains they contain. LIM domains are highly conserved cysteine-rich str ...; MIM 601329). The SSH family appears to play a role in actin dynamics by reactivating ADF/cofilin proteins in vivo (Niwa et al., 2002). upplied by OMIMref name="entrez"> References Further reading * * * * {{gene-11-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |